SUMMARY: The KRAS (Kirsten rat sarcoma viral oncogene homologue) proto-oncogene encodes a protein that is a member of the small GTPase super family. The KRAS gene provides instructions for making the KRAS protein, which is a part of a signaling pathway known as the RAS/MAPK pathway. By relaying signals from outside the cell to the cell nucleus, the protein instructs the cell to grow, divide and differentiate. The KRAS protein is a GTPase, and converts GTP into GDP. To transmit signals, the KRAS protein must be turned on, by binding to a molecule of GTP. When GTP is converted to GDP, the KRAS protein is turned off or inactivated, and when the KRAS protein is bound to GDP, it does not relay signals to the cell nucleus. The KRAS gene is in the Ras family of oncogenes, which also includes two other genes, HRAS and NRAS. When mutated, oncogenes have the potential to change normal cells cancerous.
KRAS is the most frequently mutated oncogene in human cancers and are often associated with resistance to targeted therapies and poor outcomes. The KRAS-G12C mutation occurs in approximately 12-15% of Non Small Cell Lung Cancers (NSCLC) and in 3-5% of Colorectal cancers and other solid cancers. G12C is a single point mutation with a Glycine-to-Cysteine substitution at codon 12. This substitution favors the activated state of KRAS, resulting in a predominantly GTP-bound KRAS oncoprotein, amplifying signaling pathways that lead to oncogenesis.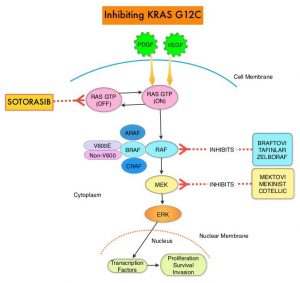
Sotorasib (AMG 510) is a small molecule that specifically and irreversibly inhibits KRAS-G12C and traps KRAS-G12C in the inactive GDP-bound state. Preclinical studies in animal models showed that Sotorasib inhibited nearly all detectable phosphorylation of Extracellular signal-Regulated Kinase (ERK), a key downstream effector of KRAS, leading to durable complete regression of KRAS-G12C tumors.
The authors conducted a multicenter, open label Phase I trial of Sotorasib, in patients with advanced solid tumors harboring the KRAS-G12C mutation. This trial consisted of dose escalation and expansion cohorts and included a total of 129 patients, of whom 59 patients had NSCLC, 42 had Colorectal cancer, and 28 patients had other tumor types (Appendiceal, Endometrial, Pancreatic cancers and Melanoma). Sotorasib was administered orally once daily and each treatment cycle was 21 days. The planned dose levels for the escalation cohorts were 180, 360, 720, and 960 mg. Treatment was continued until disease progression or unacceptable toxicity. The median patient age was 62 years and most of the enrolled patients were heavily pretreated and had received a median of 3 previous lines of anticancer therapy for metastatic disease. Among the NSCLC patient cohort, approximately 90% of patients were current or former smokers and had received anti- Programmed cell Death protein-1 (PD-1) or PD-Ligand 1 (PD-L1) therapies. All patients had received previous platinum-based chemotherapy. The Primary endpoint was safety, including the incidence of dose-limiting toxicities and key Secondary end points were pharmacokinetics and Objective Response Rates. The Sotorasib dose of 960 mg daily was identified as the dose for the expansion cohort. The median follow up was 11.7 months and the median duration of treatment was 3.9 months, with 57% of patients having received treatment for 3 months or more, and 29% of patients, for 6 months or more.
Among those patients with NSCLC, 32.2% of the patients had a confirmed Objective Response (Complete or Partial Response at all dose levels, and 88% had disease control (Objective Response or Stable disease), with a median Progression Free Survival of 6.3 months. Responses were rapid and were seen at week 6, and these responses were durable and ongoing at a median follow up of nearly a year.
Among the colorectal cancer subgroup, at a median follow up of 12.8 months, 7% had a confirmed response, and 74% had disease control, with a median duration of stable disease of 5.4 months and median PFS of 4 months. Responses were also observed in patients with Pancreatic, Endometrial, and Appendiceal cancers and Melanoma. It has been postulated that the inconsistent tumor responses noted between NSCLC and Colorectal cancer suggests either that KRAS-G12C is not the dominant oncogenic driver for colorectal cancer or that other pathways such as Wnt or EGFR pathways may mediate oncogenic signaling beyond KRAS. The authors suggest that a viable option would be to combine Sotorasib with therapies that block additional pathways, as was shown by studies in BRAF V600E-mutant Colorectal cancer. Approximately 57% of patients had treatment-related Adverse Events, of whom, about 12% had Grade 3 or 4 events. These toxicities included abnormal liver function studies, anemia, lymphopenia and diarrhea.
It was concluded Sotorasib showed promising anticancer activity in patients with heavily pretreated advanced solid tumors harboring the KRAS-G12C mutation. Studies evaluating Sotorasib as monotherapy or in combination with various agents in patients with NSCLC or other solid tumors are under way
KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. Hong DS, Fakih MG, Strickler JH, et al. N Engl J Med 2020; 383:1207-1217.

