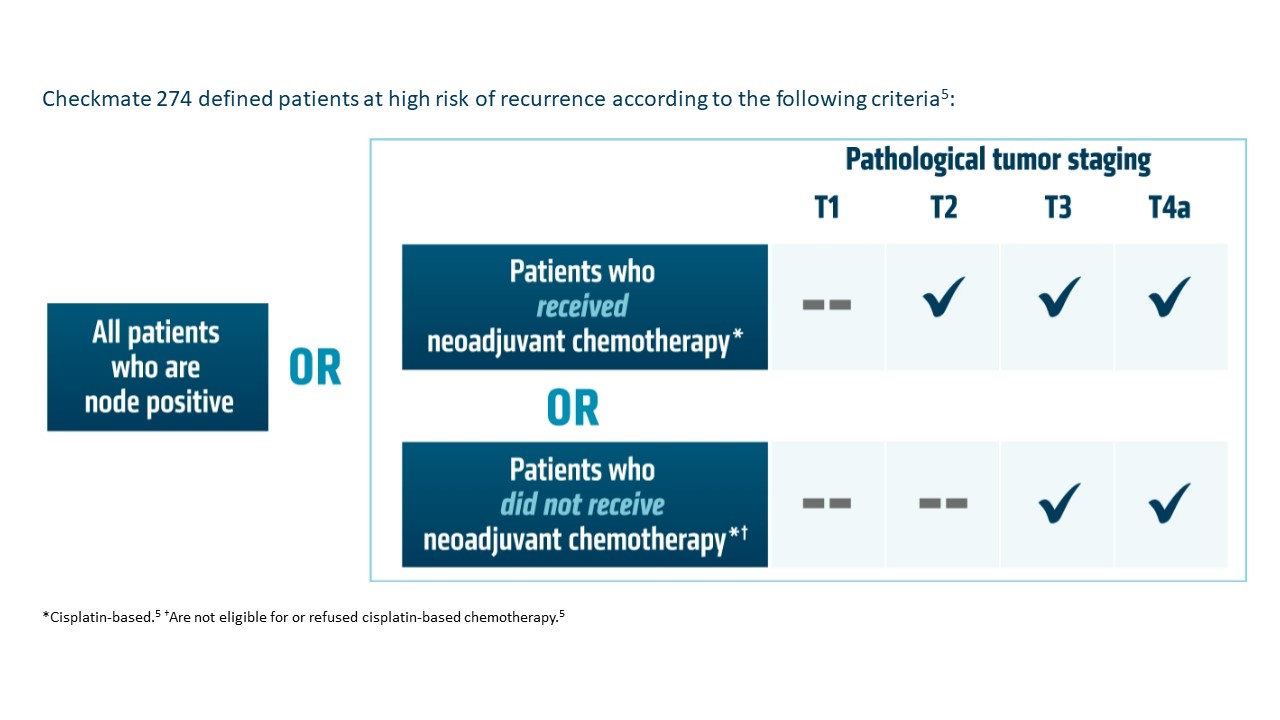
*Urothelial carcinoma at high risk of recurrence after undergoing radical resection.
Written by: Terence Friedlander, MD
Professor of Medicine, Division of Hematology/Oncology, Zuckerberg San Francisco General Hospital, Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco
Content sponsored by: Bristol Myers Squibb
Dr Friedlander is a paid consultant for BMS and was compensated for his contribution in drafting this content.
Overview of High-Risk Urothelial Carcinoma*
Currently, radical resection with or without perioperative therapy is the standard of care for treating high-risk urothelial carcinoma (UC).1* However, there is still a high chance of recurrence within 2 years of radical resection, with less favorable survival rates for the high-risk patient population.1 While neoadjuvant therapy has an established role in treating high-risk UC,* data are less clear regarding the role of adjuvant therapy.2 In a retrospective observational cohort study of patients 65 years or older with UC at high risk of recurrence after radical resection, including patients who received neoadjuvant chemotherapy, median disease-free survival (mDFS) was determined to be 13.5 months.1 Cisplatin-based chemotherapy is the neoadjuvant standard of care, but prior to 2021 there were no FDA-approved adjuvant therapy options.1-3 Studies have shown that adjuvant chemotherapy may delay recurrence and improve overall survival (OS), but these studies have not definitively shown a survival benefit, largely due to inadequate sample sizes.2 Additionally, approximately 50% of patients are ineligible for cisplatin-based treatment.1 As a result, there is a high unmet need for this difficult-to-treat population, and it is important for the urologist, oncologist, and patient to discuss and align on perioperative treatments at the time of diagnosis and early in the patient journey.1,2,4 Entering the adjuvant treatment landscape, immune checkpoint inhibitors may be an additional treatment option for HCPs to consider for their patients with high-risk UC.1,2*
Adjuvant OPDIVO in High-Risk Urothelial Carcinoma*
OPDIVO is approved and indicated for the adjuvant treatment of adult patients with UC who are at high risk of recurrence after undergoing radical resection, regardless of prior neoadjuvant chemotherapy, nodal involvement, or PD-L1 status.5 The approval is based on Checkmate 274, a phase 3, multicenter, double-blind, randomized trial of adjuvant OPDIVO versus placebo.6 More information on the study design can be found in the images below. Baseline characteristics were balanced across treatment arms.6
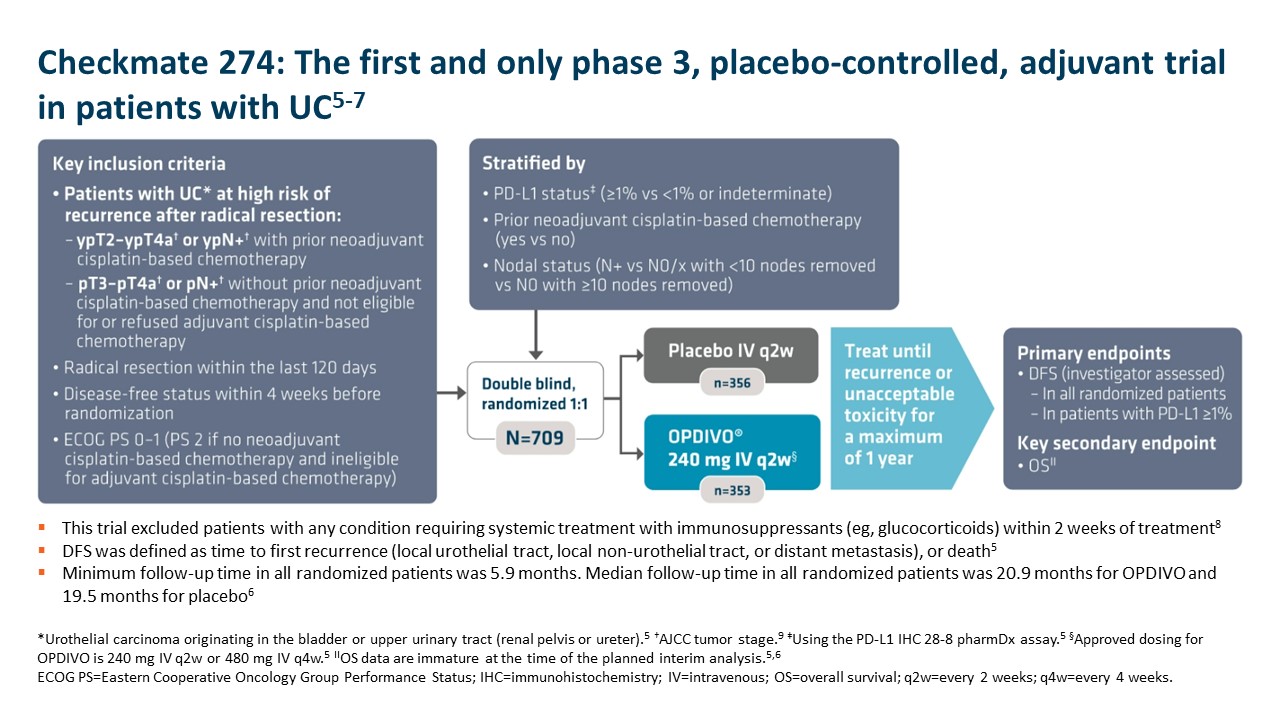 Important Safety Information
Important Safety Information
Select Important Safety Information
In Checkmate 274, serious adverse reactions occurred in 30% of OPDIVO patients. The most frequent serious adverse reaction reported in ≥2% of patients was urinary tract infection. Fatal adverse reactions occurred in 1% of patients; these included events of pneumonitis (0.6%). The most-common adverse reactions reported in ≥20% of patients were rash, fatigue, diarrhea, pruritus, musculoskeletal pain, and UTI. OPDIVO was discontinued or delayed due to adverse reactions in 18% and 33% of patients, respectively.5
OPDIVO is associated with the following Warnings and Precautions: severe and fatal immune-mediated adverse reactions including pneumonitis, colitis, hepatitis and hepatotoxicity, endocrinopathies, nephritis with renal dysfunction, dermatologic adverse reactions, other immune-mediated adverse reactions; infusion-related reactions; complications of allogeneic hematopoietic stem cell transplantation; embryo-fetal toxicity; and increased mortality in patients with multiple myeloma when OPDIVO is added to a thalidomide analogue and dexamethasone, which is not recommended outside of controlled clinical trials.
OPDIVO may cause severe infusion-related reactions. In patients who received OPDIVO as a 60-minute intravenous infusion, infusion-related reactions occurred in 6.4% (127/1994) of patients.5 For additional information regarding infusion-related reactions, please see Important Safety Information for OPDIVO.
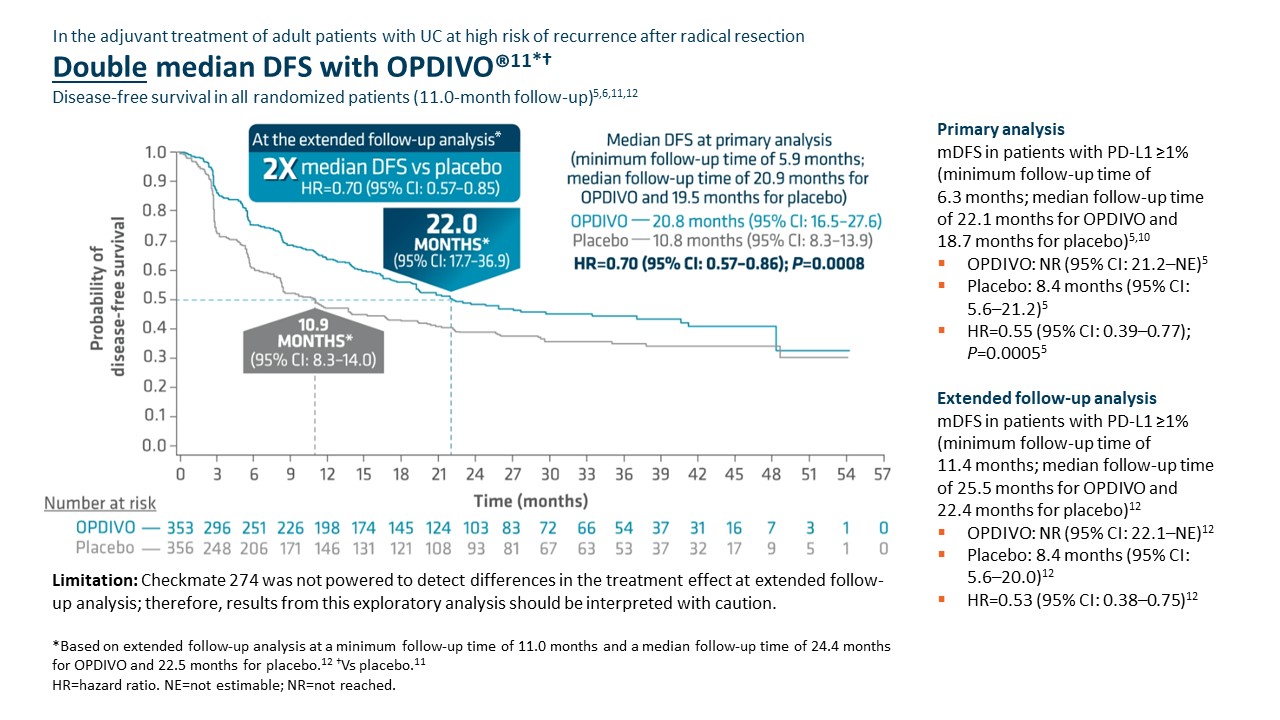 Checkmate 274 was not powered to detect differences in the treatment effect at extended follow-up analysis; therefore, results from this exploratory analysis should be interpreted with caution.
Checkmate 274 was not powered to detect differences in the treatment effect at extended follow-up analysis; therefore, results from this exploratory analysis should be interpreted with caution.
Adjuvant OPDIVO demonstrated superior disease-free survival (DFS) compared with placebo at the primary analysis (minimum follow-up of 5.9 months).5,6 Median DFS was 20.8 months with OPDIVO versus 10.8 months with placebo (HR=0.70 [95% CI: 0.57–0.86];P=0.0008).5 OS was also evaluated as a secondary endpoint, but at the time of the planned interim analysis, these data were immature with 33% of deaths in the ITT population; in the UTUC subpopulation, 37 deaths occurred, 20 of which occurred with OPDIVO versus 17 with placebo.5 Although the subgroup analyses were not statistically powered, for patients with prior neoadjuvant cisplatin therapy (n=308), the DFS hazard ratio was 0.52 [95% CI: 0.38–0.71] and for patients without prior neoadjuvant cisplatin therapy (n=401), the DFS hazard ratio was 0.92 [95% CI: 0.69–1.21].6 In additional exploratory subgroup analyses, no improvement in DFS was observed with nivolumab compared to placebo in patients with UTUC (n=149) the unstratified DFS hazard ratio was 1.15 (95% CI: 0.74–1.80); in patients with PD-L1 expression of <1% (n=414), the unstratified DFS hazard ratio was 0.83 (95% CI: 0.64–1.08).5
At the extended follow-up analysis (minimum follow-up of 11.0 months), mDFS was doubled with adjuvant OPDIVO compared with placebo. Median DFS was 22.0 months with OPDIVO versus 10.9 months with placebo (HR=0.70 [95% CI: 0.57–0.85]).12
Summary/conclusions
Given the high unmet need in this difficult-to-treat population, the call for approved adjuvant treatment options continues to rise.1,2 Adjuvant OPDIVO offers a chance to change the future for patients with high-risk UC as the only FDA-approved adjuvant option for adult patients with UC at high risk of recurrence after radical resection regardless of prior neoadjuvant chemotherapy, nodal involvement, or PD-L1 status.5,6,12 In Checkmate 274, OPDIVO significantly extended mDFS at the time of primary analysis and doubled mDFS at the time of extended follow-up analysis.5,6,12 Further data will be generated for the secondary endpoint of OS, which may provide greater insight into the efficacy of OPDIVO in this context.6,8 Given the clinical profile of Checkmate 274 and subsequent FDA approval, OPDIVO may help extend DFS for appropriate patients in need of treatment in the adjuvant UC setting.5,6,12
*Urothelial carcinoma at high risk of recurrence after undergoing radical resection.
Additional Definitions
CI=confidence interval; HCP=healthcare provider; HR=hazard ratio; ITT=intent to treat; PD-L1=programmed death ligand 1; UTUC=upper tract urothelial carcinoma.
Indication
OPDIVO® (nivolumab), as a single agent, is indicated for the adjuvant treatment of adult patients with urothelial carcinoma (UC) who are at high risk of recurrence after undergoing radical resection of UC.
Important Safety Information
Severe and Fatal Immune-Mediated Adverse Reactions
Immune-mediated adverse reactions listed herein may not include all possible severe and fatal immune-mediated adverse reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. While immune-mediated adverse reactions usually manifest during treatment, they can also occur after discontinuation of OPDIVO. Early identification and management are essential to ensure safe use of OPDIVO. Monitor for signs and symptoms that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment with OPDIVO. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue OPDIVO depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). In general, if OPDIVO interruption or discontinuation is required, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy. Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
OPDIVO can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation. In patients receiving OPDIVO monotherapy, immune-mediated pneumonitis occurred in 3.1% (61/1994) of patients, including Grade 4 (<0.1%), Grade 3 (0.9%), and Grade 2 (2.1%).
Immune-Mediated Colitis
OPDIVO can cause immune-mediated colitis. A common symptom included in the definition of colitis was diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies. In patients receiving OPDIVO monotherapy, immune-mediated colitis occurred in 2.9% (58/1994) of patients, including Grade 3 (1.7%) and Grade 2 (1%).
Immune-Mediated Hepatitis and Hepatotoxicity
OPDIVO can cause immune-mediated hepatitis. In patients receiving OPDIVO monotherapy, immune-mediated hepatitis occurred in 1.8% (35/1994) of patients, including Grade 4 (0.2%), Grade 3 (1.3%), and Grade 2 (0.4%).
Immune-Mediated Endocrinopathies
OPDIVO can cause primary or secondary adrenal insufficiency, immune-mediated hypophysitis, immune- mediated thyroid disorders, and Type 1 diabetes mellitus, which can present with diabetic ketoacidosis. Withhold OPDIVO depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism; initiate hormone replacement as clinically indicated. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism; initiate hormone replacement or medical management as clinically indicated. Monitor patients for hyperglycemia or other signs and symptoms of diabetes; initiate treatment with insulin as clinically indicated.
In patients receiving OPDIVO monotherapy, adrenal insufficiency occurred in 1% (20/1994), including Grade 3 (0.4%) and Grade 2 (0.6%).
In patients receiving OPDIVO monotherapy, hypophysitis occurred in 0.6% (12/1994) of patients, including Grade 3 (0.2%) and Grade 2 (0.3%).
In patients receiving OPDIVO monotherapy, thyroiditis occurred in 0.6% (12/1994) of patients, including Grade 2 (0.2%).
In patients receiving OPDIVO monotherapy, hyperthyroidism occurred in 2.7% (54/1994) of patients, including Grade 3 (<0.1%) and Grade 2 (1.2%).
In patients receiving OPDIVO monotherapy, hypothyroidism occurred in 8% (163/1994) of patients, including Grade 3 (0.2%) and Grade 2 (4.8%).
In patients receiving OPDIVO monotherapy, diabetes occurred in 0.9% (17/1994) of patients, including Grade 3 (0.4%) and Grade 2 (0.3%), and 2 cases of diabetic ketoacidosis.
Immune-Mediated Nephritis with Renal Dysfunction
OPDIVO can cause immune-mediated nephritis. In patients receiving OPDIVO monotherapy, immune-mediated nephritis and renal dysfunction occurred in 1.2% (23/1994) of patients, including Grade 4 (<0.1%), Grade 3 (0.5%), and Grade 2 (0.6%).
Immune-Mediated Dermatologic Adverse Reactions
OPDIVO can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS) has occurred with PD-1/PD-L1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate nonexfoliative rashes.
Withhold or permanently discontinue OPDIVO depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information).
In patients receiving OPDIVO monotherapy, immune-mediated rash occurred in 9% (171/1994) of patients, including Grade 3 (1.1%) and Grade 2 (2.2%).
Other Immune-Mediated Adverse Reactions
The following clinically significant immune-mediated adverse reactions occurred at an incidence of <1% (unless otherwise noted) in patients who received OPDIVO monotherapy or were reported with the use of other PD-1/PD- L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions: cardiac/vascular: myocarditis, pericarditis, vasculitis; nervous system: meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy; ocular: uveitis, iritis, and other ocular inflammatory toxicities can occur; gastrointestinal: pancreatitis to include increases in serum amylase and lipase levels, gastritis, duodenitis; musculoskeletal and connective tissue: myositis/polymyositis, rhabdomyolysis, and associated sequelae including renal failure, arthritis, polymyalgia rheumatica; endocrine: hypoparathyroidism; other (hematologic/immune): hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis (HLH), systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection.
Some ocular IMAR cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada–like syndrome, which has been observed in patients receiving OPDIVO, as this may require treatment with systemic corticosteroids to reduce the risk of permanent vision loss.
Infusion-Related Reactions
OPDIVO can cause severe infusion-related reactions. Discontinue OPDIVO in patients with severe (Grade 3) or life-threatening (Grade 4) infusion-related reactions. Interrupt or slow the rate of infusion in patients with mild (Grade 1) or moderate (Grade 2) infusion-related reactions. In patients receiving OPDIVO monotherapy as a 60- minute infusion, infusion-related reactions occurred in 6.4% (127/1994) of patients. In a separate trial in which patients received OPDIVO monotherapy as a 60-minute infusion or a 30-minute infusion, infusion-related reactions occurred in 2.2% (8/368) and 2.7% (10/369) of patients, respectively. Additionally, 0.5% (2/368) and 1.4% (5/369) of patients, respectively, experienced adverse reactions within 48 hours of infusion that led to dose delay, permanent discontinuation or withholding of OPDIVO.
Complications of Allogeneic Hematopoietic Stem Cell Transplantation
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with OPDIVO. Transplant-related complications include hyperacute graft-versus-host-disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between OPDIVO and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with OPDIVO prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal studies, OPDIVO can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with OPDIVO and for at least 5 months after the last dose.
Increased Mortality in Patients with Multiple Myeloma when OPDIVO is Added to a Thalidomide Analogue and Dexamethasone
In randomized clinical trials in patients with multiple myeloma, the addition of OPDIVO to a thalidomide analogue plus dexamethasone resulted in increased mortality. Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials.
Lactation
There are no data on the presence of OPDIVO in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment and for 5 months after the last dose.
Serious Adverse Reactions
In Checkmate 274, serious adverse reactions occurred in 30% of patients receiving OPDIVO (n=351). The most frequent serious adverse reaction reported in ≥2% of patients receiving OPDIVO was urinary tract infection. Fatal adverse reactions occurred in 1% of patients; these included events of pneumonitis (0.6%).
Common Adverse Reactions
In Checkmate 274, the most common adverse reactions (≥20%) reported in patients receiving OPDIVO (n=351) were rash (36%), fatigue (36%), diarrhea (30%), pruritus (30%), musculoskeletal pain (28%), and urinary tract infection (22%).
Please see US Full Prescribing Information for OPDIVO.
References
1. Drakaki A, Pantuck A, Mhatre SK, et al. “Real-world” outcomes and prognostic indicators among patients with high-risk muscle-invasive urothelial carcinoma. Urol Oncol. 2021;39:76.e15-76.e22.
2. Referenced without permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Bladder Cancer V.2.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed August 4, 2022. To view the most recent and complete version of the guidelines, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
3. Apolo AB, Msaouel P, Niglio S, et al. Evolving Role of Adjuvant Systemic Therapy for Kidney and Urothelial Cancers. Am Soc Clin Oncol Educ Book. 2022;42:1-16. doi:10.1200/EDBK_350829.
4. Nayan M, Bhindi B, Yu JL, et al. The initiation of a multidisciplinary bladder cancer clinic and the uptake of neoadjuvant chemotherapy: A time-series analysis. Can Urol Assoc J. 2016;10(1-2):25-30.
5. OPDIVO [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
6. Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384(22):2102-2114.
7. Bajorin DF, Witjes JA, Gschwend JE, et al. First results from the phase 3 CheckMate 274 trial of adjuvant nivolumab versus placebo in patients who underwent radical surgery for high-risk muscle-invasive urothelial carcinoma. Oral presentation at ASCO GU 2021. Abstract 391.
8. Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384(22):2102-2114 [supplementary appendix].
9. American Cancer Society. Bladder cancer early detection, diagnosis, and staging. Accessed August 5, 2022. https://www.cancer.org/content/dam/CRC/PDF/Public/8559.00.pdf.
10. Data on file. NIVO 639. Princeton, NJ: Bristol-Myers Squibb Company; 2021.
11. Witjes JA, Bajorin DF, Galsky MD, et al. Results for patients with muscle-invasive bladder cancer in the CheckMate 274 trial. Poster presentation at ASCO 2022. Abstract 4585.
12. Galsky MD, Witjes JA, Gschwend JE, et al. Disease-free survival with longer follow-up from the phase 3 CheckMate 274 trial of adjuvant nivolumab in patients who underwent surgery for high-risk muscle-invasive urothelial carcinoma. Oral presentation at the American Urological Association (AUA) Annual Meeting 2022. Abstract 22-3807.
© 2022 Bristol-Myers Squibb Company. OPDIVO® and the related logos are trademarks of Bristol-Myers Squibb Company. 1506-US-2200368 8/22

 Important Safety Information
Important Safety Information
