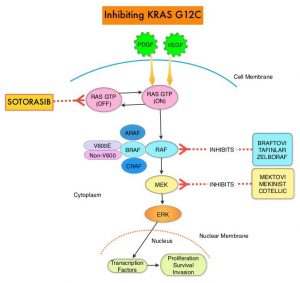
SUMMARY: ColoRectal Cancer (CRC) is the third most common cancer diagnosed in both men and women in the United States. The American Cancer Society estimates that approximately 151,030 new cases of CRC will be diagnosed in the United States in 2022 and about 52,580 patients are expected to die of the disease. The lifetime risk of developing CRC is about 1 in 23.
Approximately 15-25% of the patients with CRC present with metastatic disease at the time of diagnosis (synchronous metastases) and 50-60% of the patients with CRC will develop metastatic disease during the course of their illness. First line treatment of metastatic CRC include Oxaliplatin or Irinotecan, in combination with a Fluoropyrimidine and Leucovorin (FOLFOX or FOLFIRI), along with a VEGF targeting agent such as Bevacizumab or EGFR targeting agents such as Cetuximab and Panitumumab. However numerous studies have failed to clearly establish that any of these combination regimens would be superior for any given patient based on clinical factors. Nonetheless, majority of patients with metastatic colorectal cancer receive FOLFOX-based first line treatment in the US.
A retrospective evaluation from the Phase III 80405 clinical trial which included data from 1,025 patients with KRAS wild-type disease, concluded that the biology of tumors originating in the right colon may be different from those originating in the left colon, with Cetuximab showing superiority over Bevacizumab, when combined with chemotherapy, in KRAS wild-type patients with left-sided colon cancer. (J Clin Oncol 34, 2016: suppl; abstr 3504).
Panitumumab (VECTIBIX®) is a human IgG2 kappa monoclonal antibody, that targets and antagonizes Epidermal Growth Factor Receptor (EGFR). The PARADIGM Trial is a multicenter, open-label, prospective, Phase III study conducted in Japan, to evaluate the efficacy and superiority of mFOLFOX6 plus Panitumumab compared to mFOLFOX6 plus Bevacizumab, in the first line treatment of chemotherapy-naïve patients with RAS wild type (KRAS/NRAS gene) metastatic colorectal cancer and left-sided primary tumors (descending colon, sigmoid colon, and rectum). In this first prospective randomized study, a total of 400 patients received Panitumumab and 402 received Bevacizumab. Both groups received mFOLFOX6. Most of the patients had left sided tumors (N=614) of whom 312 patients received Panitumumab with chemotherapy, whereas 292 patients received Bevacizumab with chemotherapy. The Primary endpoint of Overall Survival (OS) was hierarchically tested in patients with left-sided tumors, followed by evaluation in the entire study population. Key Secondary endpoints included Progression Free Survival (PFS), Objective Response Rate (ORR), and curative resection (R0) rate. Overall Survival in patients with left-sided tumors was analyzed after a median follow up of 61 months.
The study met its Primary endpoint and Panitumumab in combination with mFOLFOX6 significantly improved median Overall Survival, compared to Bevacizumab plus mFOLFOX6 in the left-sided tumor population, with a 18% lower risk of death (37.9 months versus 34.3 months; HR=0.82; P=0.031). When the data was subsequently analyzed for the entire study group, the OS benefit also significantly favored Panitumumab combination over Bevacizumab combination (median 36.2 months versus 31.3 months; HR=0.84; P=0.030). This difference however appears to be driven by the left-sided tumor population, as there was no significant OS improvement seen for patients with right-sided tumors in an exploratory analysis (median 20.2 months versus 23.2 months; HR=1.09).
There was no significant difference in the median PFS between treatment groups in the population with left-sided tumors and the median PFS was 13.7 months with Panitumumab combination and 13.2 months with Bevacizumab combination (HR=0.98). However, both Objective Response Rate and curative (R0) resection rate was higher in the Panitumumab group compared with Bevacizumab group, in the population with left-sided tumors. The Objective Response Rate was 80.2% versus 68.6%, the curative (R0) resection rate 18.3% versus 11.6% and the median duration of response was 13.1 versus 11.2 months respectively. Treatment with Panitumumab, resulted in more skin, mucosal and nail toxicities, commonly associated with EGFR inhibitors, and no new safety signal were observed.
It was concluded that in this first and largest randomized first line study comparing the efficacy of different targeted therapies in combination with standard doublet chemotherapy based on tumor sidedness, Panitumumab in combination with mFOLFOX6 significantly improved Overall Survival, resulted in a higher Objective Response Rate and a higher curative resection rate, in patients with RAS wild-type and left-sided metastatic colorectal cancer, compared with patients who received Bevacizumab plus mFOLFOX6. These findings emphasize the importance of comprehensive biomarker testing, as well as taking into consideration tumor location, in patients with metastatic colorectal cancer.
Panitumumab (PAN) plus mFOLFOX6 versus bevacizumab (BEV) plus mFOLFOX6 as first-line treatment in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC): Results from the phase 3 PARADIGM trial. Yoshino T, Watanabe J, Shitara K, et al. DOI:10.1200/JCO.2022.40.17_suppl.LBA1 Journal of Clinical Oncology 40, no. 17_suppl (June 10, 2022) LBA1.

 Conventional clinical pathological factors have
Conventional clinical pathological factors have