SUMMARY: The American Cancer Society estimates that for 2022, about 236,740 new cases of lung cancer will be diagnosed and 135,360 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of NSCLC, 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large Cell Carcinomas. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, Adenocarcinoma now is the most frequent histologic subtype of lung cancer.
The KRAS (kirsten rat sarcoma viral oncogene homologue) proto-oncogene encodes a protein that is a member of the small GTPase super family. The KRAS gene provides instructions for making the KRAS protein, which is a part of a signaling pathway known as the RAS/MAPK pathway. By relaying signals from outside the cell to the cell nucleus, the protein instructs the cell to grow, divide and differentiate. The KRAS protein is a GTPase, and converts GTP into GDP. To transmit signals, the KRAS protein must be turned on, by binding to a molecule of GTP. When GTP is converted to GDP, the KRAS protein is turned off or inactivated, and when the KRAS protein is bound to GDP, it does not relay signals to the cell nucleus. The KRAS gene is in the Ras family of oncogenes, which also includes two other genes, HRAS and NRAS. When mutated, oncogenes have the potential to change normal cells cancerous.
KRAS is the most frequently mutated oncogene in human cancers and are often associated with resistance to targeted therapies and poor outcomes. The KRAS-G12C mutation occurs in approximately 12-15% of Non Small Cell Lung Cancers (NSCLC) and in 3-5% of colorectal cancers and other solid cancers. KRAS G12C is one of the most prevalent driver mutations in NSCLC and accounts for a greater number of patients than those with ALK, ROS1, RET, and TRK 1/2/3 mutations combined. KRAS G12C cancers are genomically more heterogeneous and occur more frequently in current or former smokers, and are likely to be more complex genomically than EGFR mutant or ALK rearranged cancers. G12C is a single point mutation with a Glycine-to-Cysteine substitution at codon 12. This substitution favors the activated state of KRAS, resulting in a predominantly GTP-bound KRAS oncoprotein, amplifying signaling pathways that lead to oncogenesis.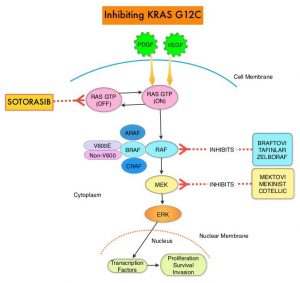
LUMAKRAS® (Sotorasib) is a first-in-class small molecule that specifically and irreversibly inhibits KRAS-G12C and traps KRAS-G12C in the inactive GDP-bound state. Preclinical studies in animal models showed that LUMAKRAS® inhibited nearly all detectable phosphorylation of Extracellular signal-Regulated Kinase (ERK), a key downstream effector of KRAS, leading to durable complete regression of KRAS-G12C tumors. The CodeBreaK clinical development program for LUMAKRAS® was designed to treat patients with an advanced solid tumor with the KRAS G12C mutation and address the longstanding unmet medical need for these cancers. This program has enrolled more than 800 patients across 13 tumor types since its inception.
CodeBreaK 100 is a Phase I and II, first-in-human, open-label, single arm, multicenter study, which enrolled patients with KRAS G12C-mutant solid tumors. Eligible patients must have received a prior line of systemic anticancer therapy, for their tumor type and stage of disease. The Phase II trial enrolled 126 patients with NSCLC, who had locally advanced or metastatic NSCLC with a KRAS G12C mutation, and had progressed on an immune checkpoint inhibitor and/or platinum-based chemotherapy. Patients with active brain metastases were excluded. Patient received LUMAKRAS® 960 mg orally once daily, until disease progression or unacceptable toxicity. The median age was 64 years, 52% were male, over 90% of patients had a smoking history, median number of prior lines of therapy was two, 92% had prior platinum-based chemotherapy and 90% had prior anti–PD-L1 therapy, 83% had both prior platinum-based chemotherapy and immunotherapy. The Primary end point of the trial was Overall Response Rate (ORR) as assessed by blinded Independent Central Review. Secondary end points included Duration of Response (DOR), Disease Control Rate (DCR), time to recovery, Progression Free Survival (PFS), Overall Survival (OS), and Safety. The examination of biomarkers served as an exploratory end point.
At the time of Primary analysis, at a median follow up of 12.2 months, the ORR was 37.1% and the median Duration of Response was 10 months. Based on the data from the primary analysis, the FDA in 2021 granted accelerated approval to LUMAKRAS®, for the treatment of patients with locally advanced or metastatic NSCLC, whose tumors harbor the KRAS G12C mutation, and who have received prior therapies.
For this updated analysis, the median follow up time for OS was 24.9 months, and the researchers included 174 patients enrolled in Phase I (N=48) and Phase II (N=126) portions of the CodeBreaK 100 trial, who were treated with LUMAKRAS®. The Overall Response Rate was 40.7% and the Disease Control Rate (DCR) was 83.7%. The median time to response was 6 weeks, the median Duration of Response was 12.3 month and 50.6% of responders remained in response for 12 months or more. The median PFS was 6.3 months and the median OS showed no change in the updated analysis, and was 12.5 months. At 1-year, the OS rate was 50.8% and the 2-year Overall Survival was 32.5%. The researchers performed additional analyses on both tumor and blood samples to identify biomarker profiles associated with durable clinical benefit and these showed that prolonged clinical benefit was observed regardless of Tumor Mutation Burden, PDL1 expression, and STK11 co-mutation status. Grade 3 or 4 treatment-related Adverse Events occurred in 21% of patients. Most adverse events were Grade 1 or 2, and treatment-related adverse events occurring in more than 10% of patients included diarrhea, elevated liver enzymes, nausea and fatigue.
It was concluded from this updated analysis that this is the longest follow up of patients on any KRAS G12C inhibitor, and LUMAKRAS® demonstrated meaningful and durable efficacy in patients with KRAS mutated NSCLC for whom treatment options are limited, following progression on first line treatment, and historically have had poor outcomes. Patients on LUMAKRAS® benefitted regardless of Tumor Mutation Burden, PDL1 expression, and STK11 co-mutation status. A global Phase III study (CodeBreaK 200) is underway, comparing LUMAKRAS® to Docetaxel in patients with KRAS G12C-mutated NSCLC.
Long-term outcomes with sotorasib in pretreated KRASp.G12C-mutated NSCLC: 2-year analysis of CodeBreaK100. Dy GK, Govindan R, Velcheti V, et al. Presented at: 2022 AACR Annual Meeting; April 8-13, 2022, New Orleans, LA. Abstract CT008.