BMS Sponsored Content
By Dr Rahul Ravilla│Sponsored by Bristol Myers Squibb
Dr Ravilla is a paid consultant for BMS and was compensated for his contribution in drafting this content.
Introduction: Overview of gastroesophageal cancers
Gastroesophageal cancers consist of a group of heterogeneous tumors, including gastric cancer (GC), gastroesophageal junction cancer (GEJC), and esophageal cancer (EC).1 The majority of GC and GEJC are adenocarcinomas, while EC is categorized into 2 main histological subtypes: esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC).2,3 EAC is the predominant histology in the United States, contributing to ~62% of all EC cases.3,4 EAC incidence rates have been increasing over the past 5 decades in Western countries.4 Recent trends in the United States also suggest increasing incidence rates of GC overall in young adults (<50 years old).5
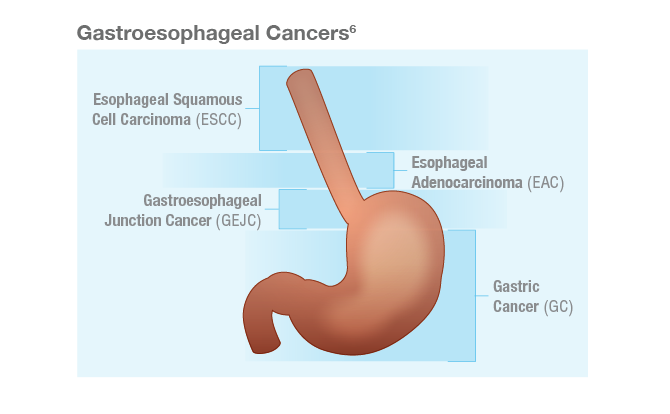
Gastric and esophageal cancers can be aggressive diseases with 5-year relative survival rates of <6% in the metastatic setting in the United States.7,8 Worldwide, GC and EC represent the fourth and sixth most common causes of cancer-related deaths, respectively.5
Approximately 15%–20% of gastroesophageal adenocarcinomas overexpress human epidermal growth factor receptor 2 (HER2)9. In this article, we will focus on HER2-negative gastroesophageal adenocarcinomas. Historically, chemotherapy has been the standard of care for the 1L treatment in this setting.10 In 2021,chemoimmunotherapy combinations were approved for appropriate patients with certain types of gastroesophageal cancers.11,12
OPDIVO + chemotherapy in 1L metastatic GC/GEJC/EAC
Currently, OPDIVO + fluoropyrimidine- and platinum-containing chemotherapy (chemo) is the only 1L chemoimmunotherapy regimen approved in metastatic non-HER2+ GC, GEJC, and EAC regardless of PD-L1 (programmed death ligand 1) status.11,13,14 The combination was approved based on the results of Checkmate 649, a global phase 3 study in 1L metastatic GC/GEJC/EAC patients with ECOG performance status 0-1.11,13 Key exclusion criteria included known HER2+ status and untreated CNS metastases.11 The study recruited all eligible patients regardless of PD-L1 expression.11,13
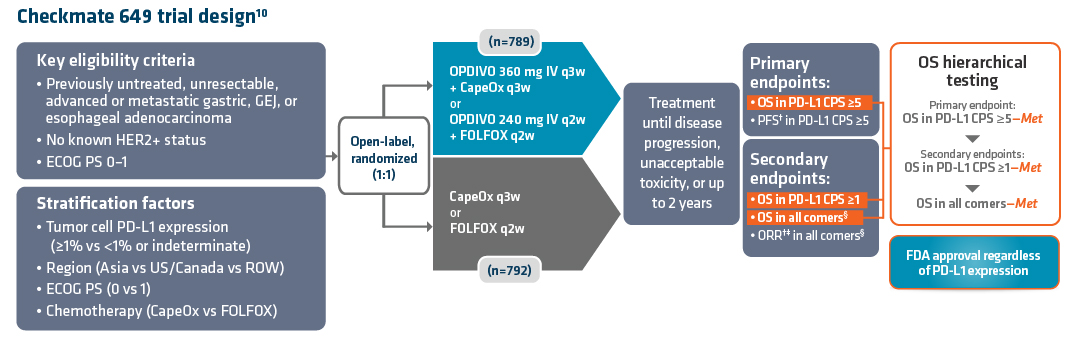
Checkmate 649 enrolled 1581 patients randomized 1:1 to OPDIVO + chemo (n=789) or chemo alone (n=792). A total of 473 patients in the OPDIVO + chemo arm and 482 patients in the chemo arm had tumors that expressed PD-L1 combined positive score (CPS) ≥5. The dual primary endpoints were overall survival (OS) and progression-free survival (PFS) in PD-L1 CPS ≥5. Key secondary endpoints included OS in PD-L1 CPS ≥1, OS in all randomized patients, and objective response rate (ORR) in all randomized patients. Checkmate 649 evaluated OPDIVO (10 mg/mL) injection for intravenous (IV) use (q2w or q3w) in combination with physician’s choice of either fluorouracil + oxaliplatin + leucovorin (mFOLFOX6) given q2w or capecitabine + oxaliplatin (CapeOx) given q3w. OPDIVO dosing was aligned with chemotherapy schedule. Treatment continued until disease progression, unacceptable toxicity, or up to 2 years. Baseline characteristics were consistent between all randomized and PD-L1 CPS ≥5 patients.13
There are Warnings and Precautions associated with OPDIVO to keep in mind. These include severe and fatal immune-mediated adverse reactions, infusion-related reactions, complications of allogeneic hematopoietic stem cell transplantation (HSCT); embryo-fetal toxicity, and increased mortality in patients with multiple myeloma when OPDIVO is added to a thalidomide analogue and dexamethasone, which is not recommended outside of controlled clinical trials.11 Additional information related to Warnings and Precautions can be found in the Important Safety Information below.
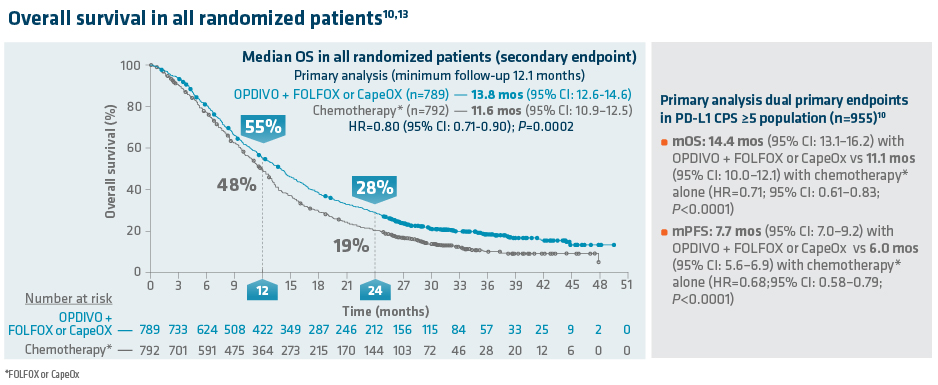
In the primary analysis (minimum[min] follow-up of 12.1 months[mos]), OPDIVO + chemo demonstrated superior OS in all randomized, PD-L1 CPS ≥1, and PD-L1 CPS ≥5 patients as compared to chemotherapy alone. In all randomized patients, mOS was 13.8 mos (95% confidence interval [CI]: 12.6–14.6) with OPDIVO + chemo vs 11.6 mos (95% CI: 10.9–12.5) with chemo (HR=0.80;95% CI: 0.71–0.90; P=0.0002). In PD-L1 CPS≥1 (n=1296), mOS was 14.0 mos (95% CI: 12.6–15.0) with OPDIVO + chemo vs 11.3 mos (95% CI: 10.6–12.3) with chemo (HR=0.77; 95% CI: 0.68–0.88; P<0.0001). In PD-L1 CPS≥5 (n=955), mOS was 14.4 mos (95% CI: 13.1–16.2) with OPDIVO + chemo vs 11.1 mos (95% CI: 10.0–12.1) with chemo (HR=0.71; 95% CI: 0.61–0.83; P<0.0001).11 The dual primary endpoint, mPFS in CPS ≥5 patients, was 7.7 mos (95% CI: 7.0–9.2) with OPDIVO + chemo vs 6.0 mos (95% CI: 5.6–6.9) with chemo (HR=0.68; 95% CI: 0.58–0.79; P<0.0001).
 *FOLFOX or CapeOx.11†Assessed using blinded independent central review (BICR).11 ‡Based on confirmed response.11§Secondary endpoint.13
*FOLFOX or CapeOx.11†Assessed using blinded independent central review (BICR).11 ‡Based on confirmed response.11§Secondary endpoint.13
Exploratory OS analyses were reported for the primary (min follow-up 12.1 months) and follow-up (min follow-up 24 months) analysis. The 12-month OS rate in all randomized patients was 55% with OPDIVO + chemo vs 48% with chemo.13 The follow-up analysis at 24.0 months reported a mOS of 13.8 mos (95% CI: 12.4–14.5) with OPDIVO + chemo vs 11.6 mos (95% CI: 10.9–12.5) with chemo in all randomized patients (HR=0.79; (95% CI: 0.71–0.88) and 14.4 mos (95% CI: 13.1–16.2) with OPDIVO + chemo vs 11.1 mos with chemo (95% CI: 10.0–12.1) in PD-L1 CPS ≥5 (HR=0.70; (95% CI: 0.60–0.81).14 The 24.0-month OS rate was 28% vs 19% for OPDIVO + chemo vs chemo, respectively, in all randomized patients.14
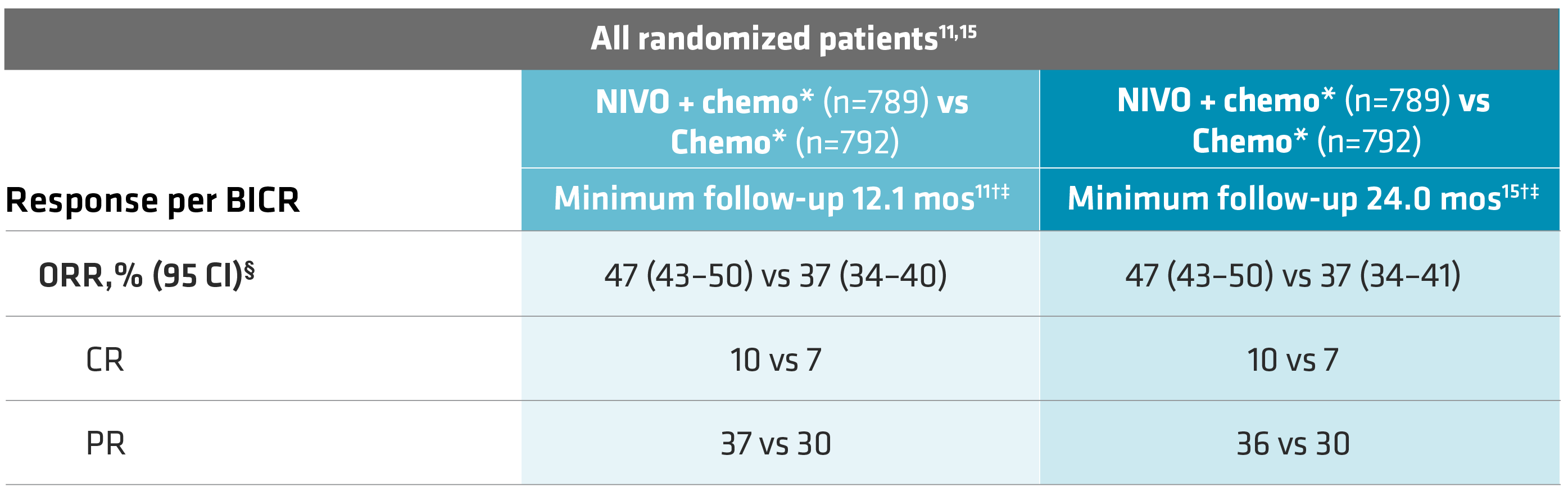
A secondary endpoint (min follow-up of 12.1 mos), ORR in all randomized patients, was 47% (95% CI: 43–50) with OPDIVO + chemo vs 37% (95% CI: 34–40) with chemo alone. Complete response (CR) rates were 10% vs 7% and partial response (PR) rates were 37% vs 30% for OPDIVO + chemo vs chemo, respectively.11
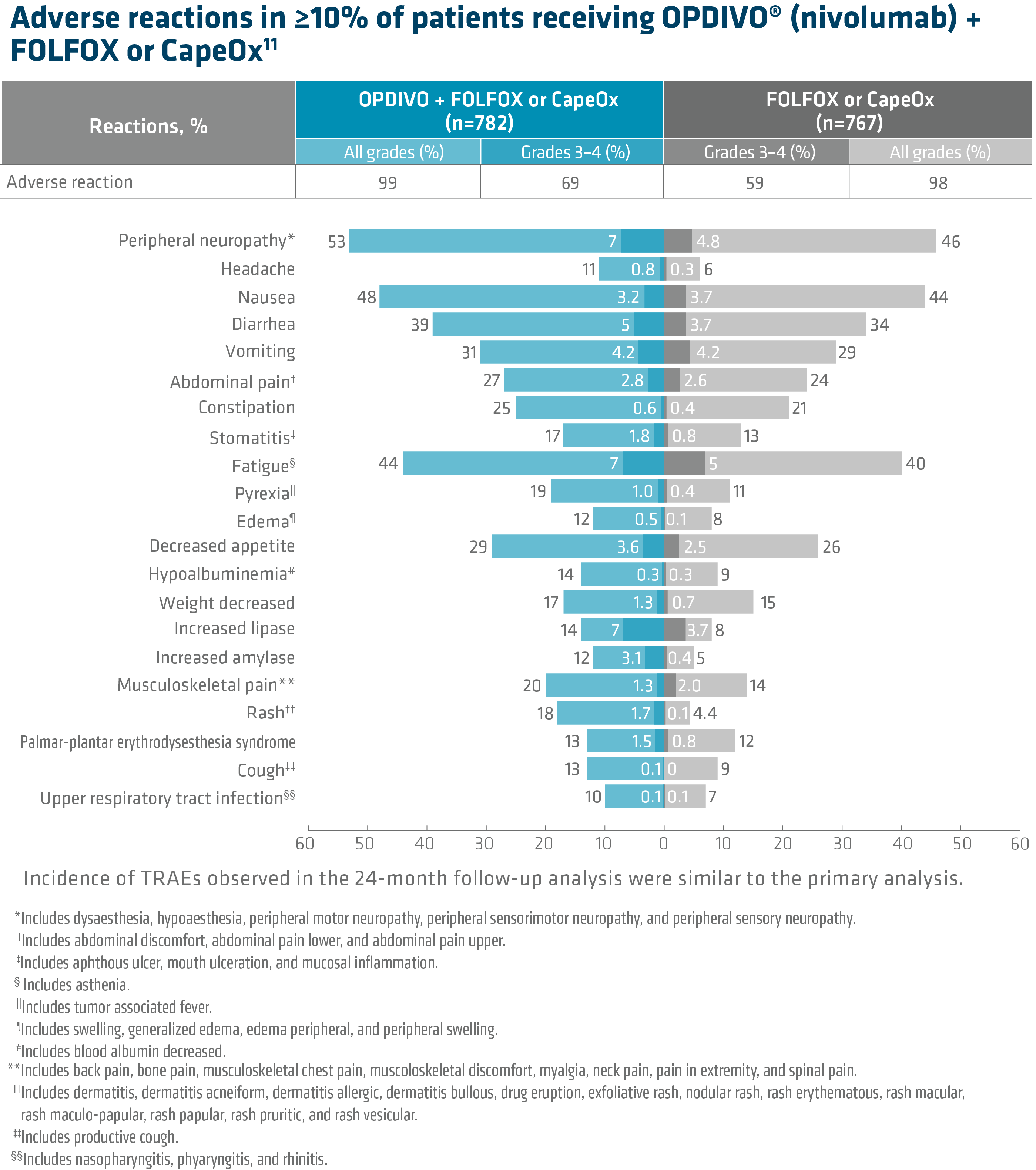
In Checkmate 649, the most common adverse reactions reported in ≥20% of patients treated with OPDIVO in combination with chemotherapy were peripheral neuropathy, nausea, fatigue, diarrhea, vomiting, decreased appetite, abdominal pain, constipation, and musculoskeletal pain. Of the ARs occurring in ≥10% of patients, those which were Grade 3–4 (OPDIVO + chemo vs chemo) were peripheral neuropathy (7% vs 4.8%), headache (0.8 vs 0.3%), nausea (3.2% vs 3.7%), diarrhea (5% vs 3.7%), vomiting (4.2% vs 4.2%), abdominal pain (2.8% vs 2.6%), constipation (0.6% vs 0.4%), stomatitis (1.8% vs 0.8%), fatigue (7% vs 5%), pyrexia (1% vs 0.4%), edema (0.5% vs 0.1%), decreased appetite (3.6% vs 2.5%), hypoalbuminemia (0.3% vs 0.3%), weight decreased (1.3% vs 0.7%), increased lipase (7% vs 3.7%), increased amylase (3.1% vs 0.4%), musculoskeletal pain (1.3% vs 2%), rash (1.7% vs 0.1%), palmar-plantar erythrodysesthesia syndrome (1.5% vs 0.8%), cough (0.1% vs 0%) and upper respiratory tract infection (0.1% vs 0.1%).
OPDIVO and/or chemotherapy were discontinued in 44% of patients and at least one dose was withheld in 76% of patients due to an adverse reaction. Serious adverse reactions occurred in 52% of patients treated with OPDIVO in combination with chemotherapy. The most frequent serious adverse reactions reported in ≥2% of patients treated with OPDIVO in combination with chemotherapy were vomiting (3.7%), pneumonia (3.6%), anemia (3.6%), pyrexia (2.8%), diarrhea (2.7%), febrile neutropenia (2.6%), and pneumonitis (2.4%). Fatal adverse reactions occurred in 16 (2.0%) patients who were treated with OPDIVO in combination with chemotherapy; these included pneumonitis (4 patients), febrile neutropenia (2 patients), stroke (2 patients), gastrointestinal toxicity, intestinal mucositis, septic shock, pneumonia, infection, gastrointestinal bleeding, mesenteric vessel thrombosis, and disseminated intravascular coagulation.11
Summary/conclusions
OPDIVO, in combination with fluoropyrimidine- and platinum-containing chemotherapy, is an approved treatment option for 1L metastatic non-HER2+ GC/GEJC/EAC regardless of PD-L1 status.11 This approval is based on the Checkmate 649 study, which at the primary analysis demonstrated superior OS with OPDIVO + chemotherapy versus chemotherapy in all randomized patients.11
1L=first line; chemo=chemotherapy; CI=confidence interval; CNS=central nervous system; ECOG=Eastern Cooperative Oncology Group; GEJC=gastroesophageal junction cancer; HR=hazard ratio; mo=month; mOS=median OS; q2w=every two weeks; q4w=every four weeks.
Indication
OPDIVO, in combination with fluoropyrimidine- and platinum-containing chemotherapy, is indicated for the treatment of patients with advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma.11
Important Safety Information
Severe and Fatal Immune-Mediated Adverse Reactions
• Immune-mediated adverse reactions listed herein may not include all possible severe and fatal immune-mediated adverse reactions.
• Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. While immune-mediated adverse reactions usually manifest during treatment, they can also occur after discontinuation of OPDIVO. Early identification and management are essential to ensure safe use of OPDIVO. Monitor for signs and symptoms that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment with OPDIVO. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
• Withhold or permanently discontinue OPDIVO depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). In general, if OPDIVO interruption or discontinuation is required, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy. Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
• OPDIVO can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation. In patients receiving OPDIVO monotherapy, immune-mediated pneumonitis occurred in 3.1% (61/1994) of patients, including Grade 4 (<0.1%), Grade 3 (0.9%), and Grade 2 (2.1%).
Immune-Mediated Colitis
• OPDIVO can cause immune-mediated colitis. A common symptom included in the definition of colitis was diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies. In patients receiving OPDIVO monotherapy, immune-mediated colitis occurred in 2.9% (58/1994) of patients, including Grade 3 (1.7%) and Grade 2 (1%).
Immune-Mediated Hepatitis and Hepatoxicity
• OPDIVO can cause immune-mediated hepatitis. In patients receiving OPDIVO monotherapy, immune-mediated hepatitis occurred in 1.8% (35/1994) of patients, including Grade 4 (0.2%), Grade 3 (1.3%), and Grade 2 (0.4%).
Immune-Mediated Endocrinopathies
• OPDIVO can cause primary or secondary adrenal insufficiency, immune-mediated hypophysitis, immune-mediated thyroid disorders, and Type 1 diabetes mellitus, which can present with diabetic ketoacidosis. Withhold OPDIVO depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism; initiate hormone replacement as clinically indicated. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism; initiate hormone replacement or medical management as clinically indicated. Monitor patients for hyperglycemia or other signs and symptoms of diabetes; initiate treatment with insulin as clinically indicated.
• In patients receiving OPDIVO monotherapy, adrenal insufficiency occurred in 1% (20/1994), including Grade 3 (0.4%) and Grade 2 (0.6%).
• In patients receiving OPDIVO monotherapy, hypophysitis occurred in 0.6% (12/1994) of patients, including Grade 3 (0.2%) and Grade 2 (0.3%).
• In patients receiving OPDIVO monotherapy, thyroiditis occurred in 0.6% (12/1994) of patients, including Grade 2 (0.2%).
• In patients receiving OPDIVO monotherapy, hyperthyroidism occurred in 2.7% (54/1994) of patients, including Grade 3 (<0.1%) and Grade 2 (1.2%).
• In patients receiving OPDIVO monotherapy, hypothyroidism occurred in 8% (163/1994) of patients, including Grade 3 (0.2%) and Grade 2 (4.8%).
• In patients receiving OPDIVO monotherapy, diabetes occurred in 0.9% (17/1994) of patients, including Grade 3 (0.4%) and Grade 2 (0.3%), and 2 cases of diabetic ketoacidosis.
Immune-Mediated Nephritis with Renal Dysfunction
• OPDIVO can cause immune-mediated nephritis. In patients receiving OPDIVO® monotherapy, immune-mediated nephritis and renal dysfunction occurred in 1.2% (23/1994) of patients, including Grade 4 (<0.1%), Grade 3 (0.5%), and Grade 2 (0.6%).
Immune-Mediated Dermatologic Adverse Reactions
• OPDIVO can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS) has occurred with PD-1/PD-L1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate nonexfoliative rashes.
• Withhold or permanently discontinue OPDIVO depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information).
• In patients receiving OPDIVO monotherapy, immune-mediated rash occurred in 9% (171/1994) of patients, including Grade 3 (1.1%) and Grade 2 (2.2%).
Other Immune-Mediated Adverse Reactions
• The following clinically significant immune-mediated adverse reactions occurred at an incidence of <1% (unless otherwise noted) in patients who received OPDIVO monotherapy or were reported with the use of other PD-1/PD-L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions: cardiac/vascular: myocarditis, pericarditis, vasculitis; nervous system: meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy; ocular: uveitis, iritis, and other ocular inflammatory toxicities can occur; gastrointestinal: pancreatitis to include increases in serum amylase and lipase levels, gastritis, duodenitis; musculoskeletal and connective tissue: myositis/polymyositis, rhabdomyolysis, and associated sequelae including renal failure, arthritis, polymyalgia rheumatica; endocrine: hypoparathyroidism; other (hematologic/immune): hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis (HLH), systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection.
• Some ocular IMAR cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada–like syndrome, which has been observed in patients receiving OPDIVO, as this may require treatment with systemic corticosteroids to reduce the risk of permanent vision loss.
Infusion-Related Reactions
• OPDIVO can cause severe infusion-related reactions. Discontinue OPDIVO in patients with severe (Grade 3) or life-threatening (Grade 4) infusion-related reactions. Interrupt or slow the rate of infusion in patients with mild (Grade 1) or moderate (Grade 2) infusion-related reactions. In patients receiving OPDIVO monotherapy as a 60-minute infusion, infusion-related reactions occurred in 6.4% (127/1994) of patients. In a separate trial in which patients received OPDIVO monotherapy as a 60-minute infusion or a 30-minute infusion, infusion-related reactions occurred in 2.2% (8/368) and 2.7% (10/369) of patients, respectively. Additionally, 0.5% (2/368) and 1.4% (5/369) of patients, respectively, experienced adverse reactions within 48 hours of infusion that led to dose delay, permanent discontinuation or withholding of OPDIVO.
Complications of Allogeneic Hematopoietic Stem Cell Transplantation
• Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with OPDIVO. Transplant-related complications include hyperacute graft-versus-host-disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between OPDIVO and allogeneic HSCT.
• Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with OPDIVO prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
• Based on its mechanism of action and findings from animal studies, OPDIVO can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with OPDIVO and for at least 5 months after the last dose.
Increased Mortality in Patients with Multiple Myeloma when OPDIVO® is Added to a Thalidomide Analogue and Dexamethasone
• In randomized clinical trials in patients with multiple myeloma, the addition of OPDIVO to a thalidomide analogue plus dexamethasone resulted in increased mortality. Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials.
Lactation
• There are no data on the presence of OPDIVO in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment and for 5 months after the last dose.
Serious Adverse Reactions
• In Checkmate 649, serious adverse reactions occurred in 52% of patients treated with OPDIVO in combination with chemotherapy (n=782). The most frequent serious adverse reactions reported in ≥2% of patients treated with OPDIVO in combination with chemotherapy were vomiting (3.7%), pneumonia (3.6%), anemia (3.6%), pyrexia (2.8%), diarrhea (2.7%), febrile neutropenia (2.6%), and pneumonitis (2.4%). Fatal adverse reactions occurred in 16 (2.0%) patients who were treated with OPDIVO in combination with chemotherapy; these included pneumonitis (4 patients), febrile neutropenia (2 patients), stroke (2 patients), gastrointestinal toxicity, intestinal mucositis, septic shock, pneumonia, infection, gastrointestinal bleeding, mesenteric vessel thrombosis, and disseminated intravascular coagulation.
Common Adverse Reactions
• In Checkmate 649, the most common adverse reactions (≥20%) in patients treated with OPDIVO in combination with chemotherapy (n=782) were peripheral neuropathy (53%), nausea (48%), fatigue (44%), diarrhea (39%), vomiting (31%), decreased appetite (29%), abdominal pain (27%), constipation (25%), and musculoskeletal pain (20%).
Please see US Full Prescribing Information for OPDIVO.
References:
1. Paydary K, Reizine N, Catenacci DVT. Immune-checkpoint inhibition in the treatment of gastro-esophageal cancer: a closer look at the emerging evidence. Cancers (Basel). 2021;13(23):5929.
2. National Cancer Institute. Gastric cancer treatment (PDQ®)–health professional version. National Cancer Institute website. Updated April 22, 2021.Accessed December 3, 2021.
http://cancer.gov/types/stomach/hp/stomach-treatment-pdq.
3. Chen Z, Ren Y, Du XL, et al. Incidence and survival differences in esophageal cancer among ethnic groups in the United States. Oncotarget. 2017;8(29):47037-47051.
4. He H, Chen N, Hou Y, et al. Trends in the incidence and survival of patients with esophageal cancer: a SEER database analysis. Thorac Cancer. 2020;11(5):1121-1128.
5. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.CA Cancer J Clin. 2021;71(3):209-249.
6. Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69(9):1564-1571.
7. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: stomach cancer. National Cancer Institute website. Accessed December 3, 2021.
http://seer.cancer.gov/statfacts/html/stomach.html.
8. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: esophageal cancer. National Cancer Institute website. Accessed December 3, 2021.
http://seer.cancer.gov/statfacts/html/esoph.html.
9. Grieb BC, Agarwal R. HER2-Directed Therapy in Advanced Gastric and Gastroesophageal Adenocarcinoma: Triumphs and Troubles. Curr Treat Options Oncol. 2021;22(10):88.
10. ShankaranV, Xiao, H, Bertwistle D, et al. A comparison of real-world treatment patterns and clinical outcomes in patients receiving first-line therapy for unresectable advanced gastric or gastroesophageal junction cancer versus esophageal adenocarcinomas. Adv Ther. 2021;38:
707-720.
11. OPDIVO® (nivolumab) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2021.
12. KEYTRUDA® (pembrolizumab) [package insert]. Kenilworth, NJ: Merck & Co., Inc; 2021.
13. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastroesophageal junction cancer/oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27-40.
14. Janjigian YY, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab vs chemotherapy as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/ esophageal adenocarcinoma: CheckMate 649 study. Presentation at ESMO 2021. Abstract LBA7.
15. Data on file. BMS-REF-NIVO-0120. Princeton, NJ: Bristol-Myers Squibb Company; 2021.
OncoPrescribeupdates@chemoforme.com
Please click here to unsubscribe. Opting out of the subscription service emails will not opt you out of messages from Bristol-Myers Squibb Company.
©2022 Bristol-Myers Squibb Company. All rights reserved. OPDIVO® and the related logos are registered trademarks of Bristol-Myers Squibb Company.
1506-US-2200006 03/22

