The FDA on August 5, 2022, approved ENHERTU® for adult patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer who have received a prior chemotherapy in the metastatic setting, or developed disease recurrence during or within six months of completing adjuvant chemotherapy. ENHERTU® is a product of Daiichi Sankyo, Inc.
Tag: Breast Cancer
Improved Outcomes with Early Switch to Fulvestrant Plus Palbociclib in ESR1 Mutated Advanced Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence. Approximately 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors. The most common subtype of metastatic breast cancer is Hormone Receptor-positive (HR-positive), HER2-negative breast cancer (65% of all metastatic breast tumors), and these patients are often treated with anti-estrogen therapy as first line treatment. However, resistance to hormonal therapy occurs in most of the patients, with a median Overall Survival (OS) of 36 months. With the development of Cyclin Dependent Kinases (CDK) 4/6 inhibitors, endocrine therapy plus a CDK4/6 inhibitor is the mainstay for the management of ER+/HER2- metastatic breast cancer as first-line therapy. Even with this therapeutic combination, most patients will eventually experience disease progression, including development of ESR1 (Estrogen Receptor gene alpha) mutations.
ESR1 is the most common acquired mutation noted in breast tumors as they progress from primary to metastatic setting. These mutations promote ligand independent Estrogen Receptor activation and have been shown to promote resistance to estrogen deprivation therapy. It appears that ESR1 mutations are harbored in metastatic ER+ breast cancers with prior Aromatase Inhibitor (AI) therapy, but not in primary breast cancers, suggesting that ESR1 mutations may be selected by prior therapy with an AI, in advanced breast cancer. In a recently published study (JAMA Oncol.2016;2:1310-1315), ESR1 mutations Y537S and D538G mutations detected in baseline plasma samples from ER+/HER- advanced breast cancer patients, was associated with shorter Overall Survival. In this study it was noted that there was a three-fold increase in the prevalence of these mutations in patients who had failed first line hormonal therapy for metastatic disease, compared with those who were initiating first line therapy for advanced breast cancer (33% versus 11%).
Fulvestrant (FASLODEX®) is an estrogen antagonist and like Tamoxifen binds to estrogen receptors (ERs) competitively, but unlike Tamoxifen causes rapid degradation and loss of ER protein (ER down regulator) and is devoid of ER agonist activity. Palbociclib (IBRANCE®) is a reversible, oral, selective, small molecule inhibitor of Cyclin Dependent Kinases, CDK4 and CDK6, and prevents RB1 phosphorylation. Palbociclib is the first CDK inhibitor approved by the FDA. It exhibits synergy when combined with endocrine therapies. The FDA in February 2016, approved Palbociclib in combination with Fulvestrant, for the treatment of women with HR-positive, HER2-negative advanced or metastatic breast cancer, with disease progression following endocrine therapy.
Patients with ESR1 mutations on Fulvestrant had improved Progression Free Survival (PFS) compared with Exemestane (AROMASIN®) in the SoFEA trial. The combination of Palbociclib and Fulvestrant improved PFS compared with Fulvestrant plus placebo in both ESR1 mutant and ESR1 wild-type patients, in the PALOMA3 trial.
The PADA-1 study aimed to show the efficacy of an early change in therapy based on a rising ESR1 mutation in the peripheral blood, while assessing the global safety of the combination Fulvestrant and Palbociclib. PADA-1 is a prospective, randomized, open-label, multicentre, Phase III trial in which 1017 patients with ER-positive, HER2-negative advanced breast cancer were included. These patients were monitored for a rising ESR1 mutation in the peripheral blood, while on first-line treatment with an Aromatase Inhibitor (Letrazole 2.5 mg, Anastrozole 1 mg or Exemestane 25 mg orally once daily, taken continuously) and Palbociclib 125 mg orally once daily on days 1-21 of a 28-day treatment cycle, at enrollment and every 2 months thereafter. Blood samples were monitored for several ESR1 mutations which included E380, P535, L536, Y537, and D538. The median time from trial enrollment to detection of the ESR1 mutation was 14.2 months. Patients with newly present or rising ESR1 mutation in the peripheral blood circulating tumor DNA and no synchronous disease progression (N=172) were randomly assigned (1:1) to continue with the same therapy (N=84) or to switch to Fulvestrant 500 mg IM on day 1 of each 28-day cycle and on day 15 of cycle 1, along with Palbociclib as previously dosed (N=88). Baseline characteristics were similar in both treatment groups. The median patient age was 61 years, and one-third of patients had prior treatment with an Aromatase Inhibitor. Patients were stratified according to visceral involvement (present or absent) and the time from inclusion to detection of ESR1 mutation in the peripheral blood (<12 months or 12 months or more). The co-Primary endpoints were Progression Free Survival and Grade 3 or more hematologic adverse events in all patients.
At a median follow up of 26.0 months from randomization, switching patients from an Aromatase Inhibitor to Fulvestrant, upon detection of ESR1 mutation in the peripheral blood was associated with a 39% reduction in the risk of disease progression or death. The median Progression Free Survival was 11.9 months in the Fulvestrant and Palbociclib group versus 5.7 months in the Aromatase Inhibitor and Palbociclib group (HR=0.61; P=0·004). The co-Primary endpoint of Grade 3 or more hematologic adverse events found no safety signals associated with switching from an Aromatase inhibitor to Fulvestrant. The most frequent Grade 3 or more hematological adverse events were neutropenia. lymphopenia, and thrombocytopenia. Dose reductions were similar in both randomized treatment groups.
The authors concluded that PADA-1 is the first prospective randomized trial to demonstrate that early therapeutic targeting of ESR1 mutation in the peripheral blood results in significant clinical benefit. The researchers added that the original design explored in PADA-1 might help with addressing acquired resistance to new drugs in future trials.
Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Bidard FC, Hardy-Bessard AC, Dalenc F, et al. The Lancet Oncology. Published: September 29, 2022.DOI:https://doi.org/10.1016/S1470-2045(22)00555-1
Late Breaking Abstract – ESMO 2022: Abemaciclib plus Transtuzumab versus Chemotherapy in HR-positive, HER2-positive Advanced Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence. Breast cancer is a heterogeneous disease and approximately 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors. The most common subtype of metastatic breast cancer is Hormone Receptor-positive (HR-positive), HER2-negative breast cancer (65% of all metastatic breast tumors), and these patients are often treated with anti-estrogen therapy as first line treatment. Human Epidermal growth factor Receptor 2 (HER2) overexpression is reported in about 15%-20% of primary breast carcinomas and is associated with poor prognosis, and nearly half of HER2-positive breast cancers are also HR-positive. Patients with HER2-positive breast cancers are generally treated with HER2-targeted therapy combined with chemotherapy. Patients with HER2-positive and HR-positive breast cancer are additionally treated with long-term hormone therapy.
Cyclin Dependent Kinases (CDKs) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. VERZENIO® (Abemaciclib) is an oral, selective inhibitor of CDK4 and CDK6 kinase activity, and prevents the phosphorylation and subsequent inactivation of the Rb tumor suppressor protein, thereby inducing G1 cell cycle arrest and inhibition of cell proliferation. HERCEPTIN® (Trastuzumab) is a humanized monoclonal antibody targeting HER2 oncogene. FASLODEX® (Fulvestrant) is a parenteral, Selective Estrogen Receptor Degrader (SERD) and is approved for the treatment of postmenopausal women with HR-positive metastatic breast cancer.
monarcHER (NCT02675231) is an International, randomized, multicenter, open-label, three-group, Phase 2 trial, conducted to compare the efficacy of Abemaciclib plus Trastuzumab with or without Fulvestrant, with standard-of-care chemotherapy of physician’s choice plus trastuzumab, in women with advanced breast cancer. In this study, 237 patients were enrolled. Eligible patients had Hormone Receptor (HR)-positive, HER2-positive advanced breast cancer, with unresectable, locally advanced, recurrent, or metastatic disease, and had previously received at least two HER2-targeted therapies for advanced disease. Patients were randomly assigned 1:1:1 to Group A (Abemaciclib, Trastuzumab, and Fulvestrant) N=79, Group B (Abemaciclib and Trastuzumab) N=79, or Group C (standard-of-care chemotherapy and trastuzumab) N=79. Treatment consisted of Abemaciclib 150 mg orally twice daily on days 1-21 of a 21-day cycle, Trastuzumab 8 mg/kg IV on cycle 1, day 1, followed by 6 mg/kg IV on day 1 of each subsequent 21-day cycle, and Fulvestrant 500 mg IM on days 1, 15, and 29 and once every 4 weeks thereafter. Standard-of-care chemotherapy was administered as specified by the product label. Patients were stratified by number of previous systemic therapies for advanced breast cancer and measurable versus non-measurable disease. An exploratory biomarker analysis of breast cancer molecular subtypes was conducted by RNA sequencing. The Primary endpoint was investigator-assessed Progression Free Survival (PFS), first testing Group A versus Group C, and if this result was significant, then Group B versus Group C. Secondary end points included Overall Survival (OS), Overall Response Rate, Patient Reported Outcomes, and pharmacokinetics. Safety was assessed in all patients who had received at least one dose of study treatment.
Previous analyses from this trial revealed that after a median follow up of 19.0 months, the Primary endpoint was met, with significantly superior PFS in Group A compared to Group C (8.3 months versus 5.7 months, respectively, HR=0.67; P=0.051), with a reduction in the risk for disease progression or death of 33%. (Lancet Oncol. 2020;21:763–775). The researchers herein reported the results, after a median follow up of 52.9 months.
The median Overall Survival was 31.1 months in Group A, 29.2 months in Group B and 20.7 months in Group C. When Group A was compared with Group C, the triplet regimen with Abemaciclib, Trastuzumab, and Fulvestrant (Group A) induced a statistically significant improvement in Overall Survival, compared with Trastuzumab plus chemotherapy (Group C). There was a numerically improved Overall Survival benefit with Abemaciclib, in combination with HER2-targeted therapy (Trastuzumab) with or without hormonal therapy (Fulvestrant), compared with chemotherapy plus Trastuzumab, and there was a consistent benefit observed with the addition of Abemaciclib across all pre-specified subgroups. Updated Progression Free Survival and safety findings were consistent with the primary analysis. An exploratory biomarker analysis by RNA sequencing suggested that Luminal subtypes were associated with longer Progression Free Survival (8.6 versus 5.4 months, HR=0.54) and Overall Survival (31.7 versus 19.7 months, HR=0.68), compared to non-Luminal subtypes. The most common serious adverse events in Group A were pyrexia, diarrhea, urinary tract infection, and acute kidney injury (3% each); in Group B were diarrhea and pneumonitis (3% each); and in Group C were neutropenia (6%) and pleural effusion (3%).
The authors concluded that based on this final analysis, a triple-agent, chemotherapy-free treatment regimen consisting of Abemaciclib plus Trastuzumab, with or without Fulvestrant, numerically improved Overall Survival in women with Hormone Receptor-positive, HER2-positive, advanced breast cancer, compared to chemotherapy plus Trastuzumab.
Final overall survival (OS) for abemaciclib plus trastuzumab +/- fulvestrant versus trastuzumab plus chemotherapy in patients with HR+, HER2+ advanced breast cancer (monarcHER): A randomized, open-label, phase II trial. Andre F, Nadal JC, Denys H, et al. Annals of Oncology (2022) 33 (suppl_7): S808-S869. 10.1016/annonc/annonc1089. LBA18
Elacestrant in ER-Positive, HER2-Negative, Metastatic Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence. Approximately 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors. The most common subtype of metastatic breast cancer is Hormone Receptor-positive (HR-positive), HER2-negative breast cancer (65% of all metastatic breast tumors), and these patients are often treated with anti-estrogen therapy as first line treatment. However, resistance to hormonal therapy occurs in a majority of the patients, with a median Overall Survival (OS) of 36 months. With the development of Cyclin Dependent Kinases (CDK) 4/6 inhibitors, endocrine therapy plus a CDK4/6 inhibitor is the mainstay, for the management of ER+/HER2-negative metastatic breast cancer, as first line therapy. Even with this therapeutic combination, most patients will eventually experience disease progression, including the development of ESR1 (Estrogen Receptor gene alpha) mutations.
ESR1 is the most common acquired mutation noted in breast tumors as they progress from primary to metastatic setting. These mutations promote ligand independent Estrogen Receptor activation and have been shown to promote resistance to estrogen deprivation therapy. It appears that ESR1 mutations are harbored in metastatic ER-positive breast cancers with prior Aromatase Inhibitor (AI) therapy, but not in primary breast cancers, suggesting that ESR1 mutations may be selected by prior therapy with an AI in advanced breast cancer. In a previously published study (JAMA Oncol.2016;2:1310-1315), ESR1 mutations Y537S and D538G mutations detected in baseline plasma samples from ER+/HER- advanced breast cancer patients, was associated with shorter Overall Survival. In this study it was noted that there was a three-fold increase in the prevalence of these mutations in patients who had failed first line hormonal therapy for metastatic disease, compared with those who were initiating first line therapy for advanced breast cancer (33% versus 11%).
Fulvestrant is a parenteral, Selective Estrogen Receptor Degrader (SERD) and is the only SERD approved for the treatment of postmenopausal women with HR-positive metastatic breast cancer. However, acquired ESR1 mutations can also occur following Fulvestrant treatment, possibly because of poor bioavailability and incomplete ER blockade when administered intramuscularly. There is therefore an urgent unmet need for an alternate SERD that has activity in tumors harboring ESR1 mutations, and has improved bioavailability allowing oral administration.
Elacestrant is an oral, nonsteroidal, Selective Estrogen Receptor Degrader (SERD) that degrades the Estrogen Receptor (ER) in a dose-dependent manner and inhibits estradiol-dependent functions of ER target gene transcription induction and breast cancer cell proliferation. Estradiol-stimulated tumor growth was diminished by Elacestrant in the ER+ xenograft models derived from heavily pretreated patients, including models resistant to CDK 4/6 inhibitors, Fulvestrant and those harboring ESR1 mutations Y537S and D538G. In an early Phase I trial, Elacestrant was noted to have an acceptable safety profile, and demonstrated single-agent activity with confirmed Partial Responses in heavily pretreated patients with ER+ metastatic breast cancer.
EMERALD trial is a multicenter, International, randomized, open-label, Phase III study, designed to evaluate the benefit of Elacestrant in patients with ER+/HER2- advanced or metastatic breast cancer. In this study, 477 postmenopausal women with ER+/HER2- metastatic breast cancer were randomly assigned 1:1 to receive either Elacestrant 400 mg orally daily (N=239) or the Standard of Care which included investigator’s choice of Fulvestrant or an Aromatase Inhibitor including Anastrozole, Letrozole, or Exemestane (N=238). Treatment was given until disease progression. Both treatment groups were well balanced. The median patient age was 63 years, and patients must have progressed or relapsed on or after 1 or 2 lines of endocrine therapy for advanced disease, one of which was given in combination with a CDK4/6 inhibitor, had 1 or fewer lines of chemotherapy for advanced disease, and had an ECOG performance status of 0 or 1. In the study, 48% had tumors with mutated ESR1 and 43% received two prior endocrine therapies. These patients were evenly distributed in both treatment groups. Patients were stratified by ESR1-mutation status, prior treatment with Fulvestrant, and visceral metastases. The co-Primary end points were Progression Free Survival (PFS) in the overall population, and in those with ESR1 mutations. Overall Survival (OS) was a Secondary end point.
Treatment with Elacestrant resulted in a statistically significant and clinically meaningful improvement in PFS, compared with Standard of Care. There was a 30% reduction in the risk of progression or death in the Elacestrant group for all patients (HR=0.70; P=0.002) and a 45% reduction in the risk of progression or death among those with ESR1 mutations (HR=0.55; P=0.0005). The researchers in this study used landmark analysis of PFS at 6 months and 12 months which selects for patients who are still sensitive to endocrine therapy and addresses the limited PFS benefit caused by an initial progression, in patients with complete endocrine resistance who do not respond to endocrine therapy. The PFS at 12 months with Elacestrant was 22.3% in all patients compared with 9.4% for those receiving the Standard of Care treatment. Among the ESR1 mutation group, the 12 month PFS rate was more pronounced and was 26.8% with Elacestrant, compared to 8.2% with Standard of Care. The benefits with Elacestrant compared with Standard of Care, was consistent across multiple prespecified subgroups including patients who had received prior Fulvestrant. There also was a trend toward improved Overall Survival in patients who received Elacestrant, compared with Standard of Care. The final Overall Survival data were not mature at the time of this analysis. Nausea of any grade occurred in 35% of patients receiving Elacestrant and 18.8% receiving Standard of Care treatment, and treatment discontinuations due to adverse events were 3.4% in the Elacestrant group versus 0.9% in the Standard of Care group.
It was concluded that Elacestrant is the first oral Selective Estrogen Receptor Degrader that demonstrated significant and clinically meaningful improvement in PFS, compared with Standard of Care endocrine therapy, in patients with ER+/ HER2- metastatic breast cancer, in the second/third line after treatment with a CDK4/6 inhibitor, and has the potential to become the new standard of care in this study population.
Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. Bidard F-C, Kaklamani VG, Neven P, et al. DOI: 10.1200/JCO.22.00338 Journal of Clinical Oncology. Published online May 18, 2022.
ENHERTU® (fam-trastuzumab deruxtecan-nxki)
The FDA on May 4, 2022, approved ENHERTU® for adult patients with unresectable or metastatic HER2-positive breast cancer, who have received a prior anti-HER2-based regimen either in the metastatic setting, or in the neoadjuvant or adjuvant setting, and have developed disease recurrence during or within 6 months of completing therapy. ENHERTU® is a product of Daiichi Sankyo, Inc.
Late Breaking Abstract – ASCO 2022: ENHERTU® for HER2-Low Advanced Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence.
It is estimated that approximately 60% of metastatic breast cancers categorized as HER2-negative express low levels of HER2, defined as a score of 1+ on ImmunoHistoChemical (IHC) analysis or as an IHC score of 2+ and negative results on In Situ Hybridization (ISH). These HER2-low breast cancer tumors are treated as HER2-negative, as currently available HER2-directed therapies have resulted in poor outcomes. These patients have limited targeted treatment options and are often treated with single agent palliative chemotherapy following progression on first line chemotherapy.
ENHERTU® (Trastuzumab Deruxtecan) is an Antibody-Drug Conjugate (ADC) composed of a humanized monoclonal antibody specifically targeting HER2, with the amino acid sequence similar to Trastuzumab, a cleavable tetrapeptide-based linker, and a potent cytotoxic Topoisomerase I inhibitor as the cytotoxic drug (payload). ENHERTU® has a favorable pharmacokinetic profile and the tetrapeptide-based linker is stable in the plasma and is selectively cleaved by cathepsins that are up-regulated in tumor cells. Unlike KADCYLA® (ado-Trastuzumab emtansine), ENHERTU® has a higher drug-to-antibody ratio (8 versus 4), released payload easily crosses the cell membrane with resulting potent cytotoxic effect on neighboring tumor cells regardless of target expression, and the released cytotoxic agent (payload) has a short half-life , thus minimizing systemic exposure. The potential activity of ENHERTU® in HER2-low breast cancer tumors is driven by the bystander antitumor effect, offered by the optimized ADC technology. Previously published Phase I and II trials have shown that ENHERTU® in heavily pretreated patients with HER2-low metastatic breast cancer resulted in an Overall Response Rate of 37%, and median Progression Free Survival ranging from 6.3 to 11.1 months.
DESTINY-Breast04 is a multicenter, randomized, open-label, Phase III trial, conducted to evaluate the efficacy and safety of ENHERTU® as compared with the physician’s choice of chemotherapy, in patients with HER2-low metastatic breast cancer. In this study, patients were randomly assigned in a 2:1 ratio to receive ENHERTU® 5.4 mg/kg IV every 3 weeks (N=373) or the physician’s choice of Capecitabine, Eribulin, Gemcitabine, Paclitaxel, or Nab-paclitaxel (N=184). Low expression of HER2 was defined as a score of 1+ on ImmunoHistoChemical (IHC) analysis or as an IHC score of 2+ and negative results on In Situ Hybridization (ISH). Randomization was stratified according to HER2-low status (IHC 1+ versus IHC 2+ and ISH-negative), the number of previous lines of chemotherapy for metastatic disease (one versus two), and Hormone Receptor (HR) status (positive versus negative) and if positive, previous CDK4/6 inhibitor therapy versus no CDK4/6 inhibitor therapy. IHC scores for HER2 expression were determined through central testing with the use of VENTANA HER2/neu investigational assay system, according to an algorithm adapted from the 2018 ASCO/CAP testing guidelines. Eligible patients must have received chemotherapy for metastatic disease or have had disease recurrence during or within 6 months after completing adjuvant chemotherapy. Patients with Hormone Receptor positive (HR-positive) disease must have received at least one line of endocrine therapy. Patients with treated, stable brain metastases were eligible. Patients were ineligible if they had a history of noninfectious interstitial lung disease treated with steroids or had suspected interstitial lung disease on imaging at screening. Both treatment groups were well balanced and approximately 89% in the ENHERTU® group and 90% in the chemotherapy group were HR-positive. The Primary end point was Progression Free Survival (PFS) among patients with HR-positive disease. Secondary end points included PFS among all patients, Overall Survival (OS) in the HR-positive cohort and among all patients, Objective Response Rate (ORR), Duration of Response, and efficacy in the HR-negative cohort. The median duration of follow up for survival was 18.4 months.
At the time of the primary efficacy analysis, the median PFS in the HR-positive cohort was 10.1 months in the ENHERTU® group and 5.4 months in the physician’s choice group (HR for disease progression or death=0.51; P<0.001). This benefit with ENHERTU® was seen consistently across all analyzed subgroups which included HER2 IHC 1+, HER2 IHC 2+ and ISH-negative, as well as those who had received previous treatment with CDK4/6 inhibitors. The median PFS among all patients was 9.9 months in the ENHERTU® group and 5.1 months in the physician’s choice group (HR for disease progression or death=0.50; P<0.001). The median PFS in the HR-negative cohort was 8.5 months in the ENHERTU® group and 2.9 months in the physician’s choice group (HR=0.46).
The median OS in the HR-positive cohort was 23.9 months in the ENHERTU® group and 17.5 months in the physician’s choice group (HR for death=0.64; P=0.003). The median OS among all patients was 23.4 months in the ENHERTU® group and 16.8 months in the physician’s choice group HR=0.64; P=0.001). The median OS in the HR-negative cohort was 18.2 months in the ENHERTU® group and 8.3 months in the physician’s choice group (HR=0.48).
The ORR in the HR-positive group was 52.6% in the ENHERTU® group and 16.3% in the physician’s choice group, and the median duration of response was 10.7 months in the ENHERTU® group and 6.8 months in the physician’s choice group. The ORR among all patients was 52.3% in the ENHERTU® group and 16.3% in the physician’s choice group. Among HR-negative cohort, the ORRs were 50% and 16.7% respectively.
Grade 3 or higher adverse events occurred in 53% of the patients who received ENHERTU® and 67.4% of those who received the physician’s choice of chemotherapy. Adjudicated, drug-related Interstitial Lung Disease or pneumonitis occurred in 12.1% of the patients who received ENHERTU®.
The authors concluded that this is the first Phase III, practice-changing trial of a HER2-directed therapy in patients with HER2-low metastatic breast cancer, to show a statistically significant and clinically meaningful benefit in PFS and OS, compared to standard chemotherapy, regardless of Hormone Receptor status, with a generally manageable safety profile. The authors added that the strong efficacy of ENHERTU® in this HER2-low patient population, with approximately 50% lower risk of disease progression and 36% lower risk of death with ENHERTU® compared to standard chemotherapy, supports the need to reclassify HER2-low as a new targetable category of metastatic breast cancer.
Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. Modi S, Jacot W, Yamashita T, et al. for the DESTINY-Breast04 Trial Investigators. N Engl J Med 2022; 387:9-20.
Late Breaking Abstract – ASCO 2022: KISQALI® with Switch Endocrine Therapy Following Progression on a Prior CDK4/6 Inhibitor in HR+/HER2-negative Metastatic Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence. Approximately 70% of breast tumors in patients with metastatic disease are Estrogen Receptor (ER) and/or Progesterone Receptor (PR) positive and HER2-negative. These patients are often treated with single agent endocrine therapy, endocrine therapy in combination with CDK4/6 inhibitor, or single agent chemotherapy.
Cyclin Dependent Kinases (CDK) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. Cyclin Dependent Kinases 4 and 6 (CDK4 and CDK6) phosphorylate RetinoBlastoma protein (RB), and initiate transition from the G1 phase to the S phase of the cell cycle. RetinoBlastoma protein has antiproliferative and tumor-suppressor activity and phosphorylation of RB protein nullifies its beneficial activities. CDK4 and CDK6 are activated in hormone receptor positive breast cancer, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation and associated with increased expression of CDK4. The understanding of the role of Cyclin Dependent Kinases in the cell cycle, has paved the way for the development of CDK inhibitors.
KISQALI® (Ribociclib) is an orally bioavailable, selective, small-molecule inhibitor of CDK4/6, preferentially inhibiting CDK4 that blocks the phosphorylation of RetinoBlastoma protein, thereby preventing cell-cycle progression and inducing G1 phase arrest.
MAINTAIN trial is an investigator-initiated, multicenter, Phase II, double-blind, placebo-controlled, prospective randomized study, conducted to evaluate the efficacy of Fulvestrant or Exemestane with or without KISQALI®, in patients with HR+/HER2-negative metastatic breast cancer, who had previously progressed on any CDK 4/6 inhibitor plus any endocrine therapy. In this study, 119 evaluable patients were randomized 1:1 to receive either KISQALI® 600mg orally once daily given 3 weeks on and 1 week off plus switch endocrine therapy (N= 60) or placebo plus switch endocrine therapy (N=59). Patients treated with prior Fulvestrant received Exemestane as endocrine therapy in the randomization, whereas if prior Exemestane was endocrine therapy, patients received Fulvestrant. If patients received neither as prior endocrine therapy, Fulvestrant or Exemestane was given per investigator discretion, although Fulvestrant was encouraged. Ultimately, 83% of patients received Fulvestrant and 17% received Exemestane. Eligible patients were postmenopausal, had HR+/HER2- negative metastatic breast cancer and had progressed on prior endocrine therapy and any CDK4/6 inhibitor. With regards to prior CDK 4/6 inhibitor treatment, 84% received IBRANCE® (Palbociclib), 11% received KISQALI®, 2% received VERZENIO® (Abemaciclib) and 3% received IBRANCE® and another CDK 4/6 inhibitor. The median duration of treatment with the prior CDK4/6 inhibitor was 15.5 months in the KISQALI® group and 17 months in the placebo group. Approximately 60% of patients had visceral metastasis, 45% had de novo metastasis at diagnosis, 18% had bone-only disease, 18% had received 2 or more prior endocrine therapies for metastatic disease, and 9% had received chemotherapy. The Primary end point was Progression Free Survival (PFS) and Secondary end points included Overall Response Rate (ORR), Clinical Benefit Rate, safety, and tumor and blood biomarkers. The median follow up was 18.2 months.
There was a statistically significant PFS improvement for patients randomized to KISQALI® plus endocrine therapy and the median PFS for patients in the KISQALI® plus endocrine therapy was 5.33 months, compared with 2.76 months for patients receiving placebo and endocrine therapy (HR=0.56;; P=0.004). At 12 months, the PFS rates were 24.6% in the KISQALI® group versus 7.4% in the placebo group. Similar results were noted in the subset of patients treated with Fulvestrant and KISQALI®, and the median PFS for those randomized to KISQALI® was 5.29 months versus 2.76 months in the placebo group (HR=0.59; P=0.02). The PFS benefit was more evident in KISQALI® group compared to the placebo group, especially among those who received a shorter duration of therapy with a prior CDK4/6 inhibitor (HR=0.36) and among those over age 65 years (HR=0.31). The Overall Response Rate in the KISQALI® group was 20%, compared to 11% in the placebo group, and the median Duration of Response was 18.8 months in those treated with KISQALI® and endocrine therapy, compared with 14.8 months for those treated with placebo plus endocrine therapy. There was also a significant improvement in the Clinical Benefit Rate (CBR), defined as patients who achieved Complete Response, Partial Response, or stable disease lasting at least 24 weeks. The CBR was significantly improved in the KISQALI® group, compared with the placebo group, and was 43% versus 25%, respectively (P=0.06). The most common adverse event in the KISQALI® group was neutropenia at 72%, compared to 15% in the placebo group.
It was concluded from this randomized, placebo-controlled trial that, treatment with KISQALI® and an alternate endocrine therapy, after progression on a prior CDK4/6 inhibitor, showed a 43% reduction in the risk of progression or death, compared with placebo and endocrine therapy, in patients with HR+/HER2-negative metastatic breast cancer.
A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin-dependent kinase 4/6 inhibition (CDK 4/6i) in patients (pts) with unresectable or hormone receptor–positive (HR+), HER2-negative metastatic breast cancer (MBC): MAINTAIN trial. Kalinsky K, Accordino MK, Chiuzan C, et al. J Clin Oncol 40, 2022 (suppl 17; abstr LBA1004). DOI: 10.1200/JCO.2022.40.17_suppl.LBA1004.
Late Breaking Abstract – ASCO 2022: Survival Benefit with TRODELVY® in Hormone Receptor Positive/HER2-Negative Metastatic Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence. Approximately 70% of breast tumors in patients with metastatic disease are Estrogen Receptor (ER) and/or Progesterone Receptor (PR) positive and HER2-negative. These patients are often treated with single agent endocrine therapy, endocrine therapy in combination with CDK4/6 inhibitor, or single agent chemotherapy. Resistance to hormonal therapy occurs in a majority of the patients and there is therefore an unmet need for agents with novel mechanisms of action.
TRODELVY® (Sacituzumab govitecan) is an Antibody-Drug Conjugate (ADC) in which SN-38, an active metabolite of Irinotecan, a Topoisomerase I inhibitor, is coupled to the humanized Anti-Trophoblast cell-surface antigen 2 (Trop-2) monoclonal antibody (hRS7 IgG1κ), through the cleavable CL2A linker. SN-38 cannot be given directly to patients because of its toxicity and poor solubility. Trop-2, a transmembrane calcium signal transducer, stimulates cancer-cell growth, and this cell surface receptor is overexpressed in several epithelial cancers including cancers of the breast, colon and lung, and has limited expression in normal human tissues. Trop-2 is expressed in more than 85% of breast tumors including Triple Negative Breast Cancer. Upon binding to Trop-2, the anti-TROP-2 monoclonal antibody is internalized and delivers SN-38 directly into the tumor cell, making it a suitable transporter for the delivery of cytotoxic drugs. Further, the cleavable linker enables SN-38 to be released both intracellularly into the tumor cells, as well as the tumor microenvironment, thereby allowing for the delivery of therapeutic concentrations of the active drug in bystander cells to which the conjugate has not bound. Thus, TRODELVY®-bound tumor cells are killed by intracellular uptake of SN-38, whereas the adjacent tumor cells are killed by the extracellular release of SN-38.
TRODELVY® was approved by the FDA in 2021 for patients with unresectable, locally advanced or metastatic Triple Negative Breast Cancer, who have received two or more prior systemic therapies, at least one of them for metastatic disease. In the IMMU-132 Phase I/II study, the Hormone Receptor positive (HR+)/HER2-negative cohort of patients with metastatic breast cancer patients had an Objective Response Rate (ORR) of 31.5%, median Progression Free Survival (PFS) of 5.5 months and median Overall Survival (OS) of 12 months, with manageable toxicities, when treated with TRODELVY®.
TROPiCS-02 is a global, open-label, randomized, Phase III study, conducted to confirm the benefit of TRODELVY® in HR+/HER2- negative advanced breast cancer. In this study, 543 patients with HR+/HER2-negative, unresectable, locally advanced or metastatic breast cancer, were randomly assigned 1:1 to receive TRODELVY® 10 mg/kg IV on D1 and 8, every 21 days (N=272), or treatment of physician’s choice, which included single agent treatment with either Capecitabine, Eribulin, Vinorelbine, or Gemcitabine (N=271). Treatment was continued until disease progression or unacceptable toxicity. Both treatment groups were well balanced. Eligible patients had 3 median prior chemotherapy regimens for metastatic breast cancer, and one prior therapy for metastatic breast cancer was allowed if disease progressed in 12 months or less after neoadjuvant chemotherapy. Patients were required to have received endocrine therapy, a CDK4/6 inhibitor and at least one prior therapy with a Taxane in any setting. Majority of patients had visceral metastases (95%), 86% had prior endocrine therapy for metastatic breast cancer for at least 6 months, and 60% and 38% received prior CDK4/6 inhibitors for 12 months or less, and for more than 12 months, respectively. The Primary endpoint was Progression Free Survival (PFS) by blinded Independent Central Review (final analysis) and key Secondary endpoint was Overall Survival (OS) at the first planned interim analysis.
The median Progression Free Survival was 5.5 months with TRODELVY® versus 4 months with standard chemotherapy (HR=0.66; P=0.0003), representing a 34% improvement in PFS with TRODELVY®. This benefit was seen across all treatment subgroups including those who were 65 years or older, those who were heavily pretreated, as well as those with visceral metastases. The Objective Response Rate (ORR) was 21% with TRODELVY® versus 14% with standard chemotherapy. The Clinical Benefit Rate was also higher with TRODELVY® versus standard chemotherapy (34% versus 22%) and median duration of response was 7.4 months and 5.6 months respectively. Overall Survival data were immature, but there was a numerical, non-significant improvement in the median Overall Survival noted in the TRODELVY® group, compared to the standard chemotherapy group (13.9 months versus 12.3 months; HR=0.84; P=0.14), respectively. Treatment with TRODELVY® also resulted in an overall health-related Quality of Life benefit over chemotherapy, with delayed deterioration in fatigue and global health status/ Quality of Life scales, according to the researchers. Grade 3 or more adverse events were observed in 74% of patients receiving TRODELVY® and in 60% of those receiving chemotherapy, and the most common toxicities associated with TRODELVY® were diarrhea and neutropenia.
It was concluded from this landmark analysis that treatment with TRODELVY® resulted in a statistically significant and clinically meaningful improvement in Progression Free Survival, compared to standard chemotherapy, in heavily pre-treated patients with HR+/HER2-negative, endocrine-resistant, unresectable, locally advanced or metastatic breast cancer, and should therefore be considered as a new treatment option for this patient population.
Primary results from TROPiCS-02: A randomized phase 3 study of sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) in patients (Pts) with hormone receptor–positive/HER2-negative (HR+/HER2-) advanced breast cancer. Rugo HS, Bardia A, Marmé F, et al. J Clin Oncol 40, 2022 (suppl 17; abstr LBA1001)
Late Breaking Abstract – ASCO 2022: Adjuvant Radiotherapy May Be Omitted in Select Patients with Luminal A Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence.
Patient undergoing breast conserving surgery, often receive adjuvant breast radiation therapy to reduce the risk of local recurrence. Radiation therapy however is inconvenient, expensive and is associated with acute and late toxicities. Previously published study by Kunkler IH, et al. (Lancet Oncol. 2015;16:266-273) concluded that radiotherapy could be avoided in a subset of elderly patients with low risk breast cancer following breast conserving surgery.
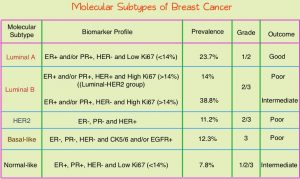 Conventional clinical pathological factors have limited ability to identify breast cancer patients with low risk disease, who could avoid radiation therapy. Molecular defined intrinsic subtypes of breast cancer can provide additional prognostic information. Breast cancer is heterogeneous malignancy and using global gene expression analyses, 5 breast cancer intrinsic subtypes have been established. They include Luminal A, Luminal B, HER2-enriched, Basal-like, and Normal breast-like group. Luminal A breast cancer patients have the lowest risk of recurrence. In a retrospective analysis of women over age 60 years, with Luminal A, Grade 1-2, T1N0 breast cancer, treated with breast conserving surgery and endocrine therapy alone, the local recurrence rate was low (JCO 2015; 33:2035). However, the utility of combining molecular subtype (Luminal A subtype) with clinical pathological factors, to guide radiotherapy decision-making, has not been prospectively evaluated.
Conventional clinical pathological factors have limited ability to identify breast cancer patients with low risk disease, who could avoid radiation therapy. Molecular defined intrinsic subtypes of breast cancer can provide additional prognostic information. Breast cancer is heterogeneous malignancy and using global gene expression analyses, 5 breast cancer intrinsic subtypes have been established. They include Luminal A, Luminal B, HER2-enriched, Basal-like, and Normal breast-like group. Luminal A breast cancer patients have the lowest risk of recurrence. In a retrospective analysis of women over age 60 years, with Luminal A, Grade 1-2, T1N0 breast cancer, treated with breast conserving surgery and endocrine therapy alone, the local recurrence rate was low (JCO 2015; 33:2035). However, the utility of combining molecular subtype (Luminal A subtype) with clinical pathological factors, to guide radiotherapy decision-making, has not been prospectively evaluated.
LUMINA is a prospective multicenter single-arm, cohort study, in which 501 women, 55 years and older, who had undergone breast conserving surgery for breast cancer, were enrolled. Eligible patients had invasive ductal T1N0, Grade 1-2, Luminal A breast cancer, had undergone breast conserving surgery, with excision margins of at least 1 mm and sentinel lymph node biopsy, omitted radiotherapy, and had received adjuvant endocrine therapy for at least 5 years. Luminal A subtype was defined as ER 1% or more, PR more than 20%, HER2 negative and Ki67 13.25% or less. Ki67 immunohistochemistry was performed centrally in one of three Canadian laboratories using International Ki67 Working Group methods. The median patient age was 67 years, 66% had Grade 1 tumors, 88% of patients were less than 75 years, and the median tumor size was 1.1 cm. Patients were followed every six months for the first two years and then yearly. The Primary outcome was local recurrence defined as time from enrollment to any invasive or non-invasive cancer in the ipsilateral breast. Secondary endpoints included contralateral breast cancer, Relapse Free Survival (RFS) based on any recurrence, Disease Free Survival, and Overall Survival.
At a median follow up of 5 years, the local recurrence rate was 2.3% and the rate of contralateral breast cancer was 1.9%. The 5-year Relapse Free Survival, Disease Free Survival and Survival rate was 97.3%, 89.9% and 97.2% respectively.
The authors concluded that among women 55 years of age and over, with low grade Luminal A breast cancer, omission of radiation therapy following breast conserving surgery and treatment with endocrine therapy alone for 5 years or more, resulted in very low rates of local recurrence at 5 years. The researchers added that approximately 30,000-40,000 women per year in North America, predominantly in the US, could avoid the morbidity, expense, and inconvenience of radiotherapy.
LUMINA: A prospective trial omitting radiotherapy (RT) following breast conserving surgery (BCS) in T1N0 luminal A breast cancer (BC). Whelan TJ, Smith S, Nielsen TO, et al. J Clin Oncol. 2022;40(suppl 17):LBA501. doi:10.1200/JCO.2022.40.17_suppl.LBA501
FDA Grants Regular Approval to ENHERTU® for Breast Cancer
SUMMARY: The FDA on May 4, 2022, approved ENHERTU® (Trastuzumab Deruxtecan) for adult patients with unresectable or metastatic HER2-positive breast cancer who have received a prior anti-HER2-based regimen either in the metastatic setting, or in the neoadjuvant or adjuvant setting and have developed disease recurrence during or within 6 months of completing therapy. In 2019, ENHERTU® received accelerated approval for adult patients with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2-based regimens in the metastatic setting. The following trial was the confirmatory trial for the accelerated approval. Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence.
The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. Patients with HER2-positive metastatic breast cancer are often treated with anti-HER2 targeted therapy along with chemotherapy, irrespective of hormone receptor status, and this has resulted in significantly improved treatment outcomes. HER2-targeted therapies include HERCEPTIN® (Trastuzumab), TYKERB® (Lapatinib), PERJETA® (Pertuzumab) and KADCYLA® (ado-Trastuzumab emtansine). Dual HER2 blockade with HERCEPTIN® and PERJETA® given along with chemotherapy (with or without endocrine therapy), as first line treatment, in HER2 positive metastatic breast cancer patients, was shown to significantly improve Progression Free Survival (PFS) as well as Overall Survival (OS). The superior benefit with dual HER2 blockade has been attributed to differing mechanisms of action and synergistic interaction between HER2 targeted therapies. Patients progressing on Dual HER2 blockade often receive KADCYLA® which results in an Objective Response Rate (ORR) of 44% and a median PFS of 9.6 months, when administered after HERCEPTIN® and a taxane. There is however no standard treatment option for this patient population following progression on KADCYLA®.
ENHERTU® (Trastuzumab Deruxtecan) is an Antibody-Drug Conjugate (ADC) composed of a humanized monoclonal antibody specifically targeting HER2, with the amino acid sequence similar to Trastuzumab, a cleavable tetrapeptide-based linker, and a potent cytotoxic Topoisomerase I inhibitor as the cytotoxic drug (payload). ENHERTU® has a favorable pharmacokinetic profile and the tetrapeptide-based linker is stable in the plasma and is selectively cleaved by cathepsins that are up-regulated in tumor cells. Unlike KADCYLA®, ENHERTU® has a higher drug-to-antibody ratio (8 versus 4), released payload easily crosses the cell membrane with resulting potent cytotoxic effect on neighboring tumor cells regardless of target expression, and the released cytotoxic agent (payload) has a short half-life , thus minimizing systemic exposure. In the DESTINY-Breast 01 Phase II registration trial involving patients with HER2-positive metastatic breast cancer, who had received two or more prior HER2 targeted therapies including KADCYLA®, the Objective Response Rate (ORR) was 60.9%, with 6% Complete Responses and 54.9% Partial Response, with a median response duration of 14.8 months. The median PFS was 16.4 months. This benefit was consistent across all key subgroups, including patients who had previously received PERJETA® therapy.
The present FDA approval was based on DESTINY-Breast 03, which is a global, multicenter, open-label, randomized Phase III study, in which the efficacy and safety of ENHERTU® was compared with KADCYLA®, in patients with HER2-positive metastatic breast cancer previously treated with Trastuzumab and a Taxane or developed disease recurrence during or within 6 months of completing neoadjuvant or adjuvant therapy. In this study, 524 pts were randomized 1:1 to receive ENHERTU® 5.4 mg/kg (N=261) or KADCYLA® 3.6 mg/kg (N=263) once every 3 weeks. Randomization was stratified by hormone receptor status, prior treatment with Pertuzumab, and history of visceral disease. The median patient age was 54 years and patients in both treatment groups were comparable in terms of baseline characteristics including age, HER2-positivity status, ECOG Performance Status, prior treatment for breast cancer, brain metastases, and prior cancer therapy with agents including Trastuzumab. The Primary endpoint was Progression Free Survival (PFS) by Blinded Independent Central Review (BICR). Secondary endpoints include Overall Survival (OS), Objective Response Rate (ORR), Duration of Response, PFS by investigator, and Safety.
At the time of the prespecified interim analysis of this study, the median follow up was approximately 16 months and the median PFS by BICR review was Not Reached with ENHERTU® and was 6.8 months with KADCYLA® (HR=0.28; P= <0.0001). This represented a very statistically significant 72% reduction in the risk for progression or death with ENHERTU® compared to KADCYLA®. The investigator-assessed PFS was similar (25.1 versus 7.2 months, HR=0.26, P<0.0001). This PFS benefit was observed as early as 4 weeks and remained consistent throughout the follow up period. PFS was significantly higher with ENHERTU® in all prespecified key subgroups, including Hormone Receptor status, prior treatment with PERJETA®, visceral disease, number of prior lines of therapy, and the presence or absence of brain metastases. Majority of patients in the ENHERTU® group experienced a reduction in tumor size, and the ORR was significantly higher among patients in the ENHERTU® compared to those who received KADCYLA® (82.7% versus 36.1%; P<0.0001), with a near doubling of the Complete Response rate in the ENHERTU® group, at 16.1% compared to 8.7% in the KADCYLA® group. The estimated 12-month Overall Survival rate was 94.1% versus 85.9% respectively (HR=0.56; P=0.007), but was not considered significant as it did not cross the prespecified boundary for significance, likely due to the immaturity of the dataset.
Adjudicated treatment related Interstitial Lung Disease/pneumonitis was more common in the ENHERTU® compared with the KADCYLA® treatment arm, at rates of 10.5% and 1.9%, respectively and most of the events were Grade 1 or 2 in severity, and none at Grade 4 or 5 in either treatment group. Interstitial Lung Disease profile was of less concern, than was seen in previous trials of ENHERTU® in more heavily pretreated patients. All Left Ventricular Ejection Fraction decreases were Grade 1 or 2 and were seen in 2.7% of the ENHERTU® group and in 0.4% of KADCYLA® group. Other serious adverse reactions in patients who received ENHERTU® included, vomiting, pyrexia, and urinary tract infection.
The researchers concluded that ENHERTU® demonstrated a highly statistically significant and clinically meaningful improvement in Progression Free Survival, when compared to KADCYLA®, in patients previously treated with Trastuzumab and Taxane for HER2-positive metastatic Breast cancer, with manageable toxicities.
Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): Results of the randomized phase III DESTINY-Breast03 study. Cortés J, Kim SB, Chung WP, et al. Presented at: European Society for Medical Oncology 2021 Virtual Congress. September 16-21, 2021; virtual. Abstract LBA1.
