The FDA on March 11, 2022, approved LYNPARZA® for the adjuvant treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) Human Epidermal growth factor Receptor 2 (HER2)-negative high-risk early breast cancer who have been treated with neoadjuvant or adjuvant chemotherapy. Patients must be selected for therapy based on an FDA-approved companion diagnostic for LYNPARZA®. LYNPARZA® is a product of AstraZeneca Pharmaceuticals, LP.
Tag: Breast Cancer
TUKYSA® in Pretreated HER2-positive Metastatic Breast Cancer With and Without Brain Metastases: Final Overall Survival Analysis
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence.
The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. Patients with HER2-positive metastatic breast cancer are often treated with anti-HER2 targeted therapy along with chemotherapy, irrespective of hormone receptor status, and this has resulted in significantly improved treatment outcomes. HER2-targeted therapies include HERCEPTIN® (Trastuzumab), TYKERB® (Lapatinib), PERJETA® (Pertuzumab), KADCYLA® (ado-Trastuzumab emtansine), ENHERTU® (Trastuzumab deruxtecan) and MARGENZA® (Margetuximab). Dual HER2 blockade with HERCEPTIN® and PERJETA®, given along with chemotherapy (with or without endocrine therapy), as first line treatment, in HER2-positive metastatic breast cancer patients, was shown to significantly improve Progression Free Survival (PFS) as well as Overall Survival (OS). The superior benefit with dual HER2 blockade has been attributed to differing mechanisms of action and synergistic interaction between HER2 targeted therapies. Patients progressing on Dual HER2 blockade often receive KADCYLA® which results in an Objective Response Rate (ORR) of 44% and a median PFS of 9.6 months, when administered after HERCEPTIN® and a taxane. There is however no standard treatment option for this patient population following progression on KADCYLA®.
It is estimated that close to 50% of patients with HER2-positive metastatic breast cancer develop brain metastases. Systemic HER2-targeted agents, including Tyrosine Kinase Inhibitors, as well as chemotherapy have limited antitumor activity in the brain. This is therefore an area of high unmet need. Local therapeutic interventions for brain metastases include neurosurgical resection and Stereotactic or Whole-Brain Radiation Therapy.
TUKYSA® (Tucatinib) is an oral Tyrosine Kinase Inhibitor that is highly selective for the kinase domain of HER2 with minimal inhibition of Epidermal Growth Factor Receptor. In a Phase 1b dose-escalation trial, TUKYSA® in combination with HERCEPTIN® and XELODA® (Capecitabine) showed encouraging antitumor activity in patients with HER2-positive metastatic breast cancer, including those with brain metastases.
HER2CLIMB is an international, randomized, double-blind, placebo-controlled trial in which the combination of TUKYSA® plus HERCEPTIN® and XELODA® was compared with placebo plus HERCEPTIN® and XELODA®. A total of 612 patients with unresectable locally advanced or metastatic HER2-positive breast cancer, who were previously treated with HERCEPTIN®, PERJETA® (Pertuzumab) and KADCYLA® (ado-Trastuzumab emtansine) were enrolled. Patients were randomly assigned in a 2:1 ratio to receive either TUKYSA® 300 mg orally twice daily throughout the treatment period (N=410) or placebo orally twice daily (N=201), in combination with HERCEPTIN® 6 mg/kg IV once every 21 days, following an initial loading dose of 8 mg/kg, and XELODA® 1000 mg/m2 orally twice daily on days 1 to 14 of each 21-day cycle. Stratification factors included presence or absence of brain metastases, ECOG Performance Status and geographic region. The median patient age was 54 years and patient demographic as well as disease characteristics at baseline were well balanced between the two treatment groups. In the total treatment population, 47.5% had brain metastases at baseline, 48.3% in the TUKYSA® combination group and 46% in the placebo combination group. The Primary endpoint was Progression Free Survival (PFS). Secondary end points included Overall Survival (OS), PFS among patients with brain metastases, confirmed Objective Response Rate (ORR), and safety.
In the primary analysis, at a median follow-up of 14 months, TUKYSA® added to HERCEPTIN® and XELODA®, significantly improved Overall Survival (OS) and Progression Free Survival (PFS) in patients with HER2-positive metastatic breast cancer. After the primary analysis, the protocol was amended to allow unblinding and cross-over from the placebo combination to the TUKYSA® combination. Protocol prespecified descriptive analyses of OS, PFS and safety were carried out at about 2 years from the last patient randomized. The researchers in this publication reported the final efficacy and safety outcomes after an additional 15.6 months follow up (total follow up of 29.6 months) in patients from the HER2CLIMB trial.
At a median follow up of 29.6 months, the median duration of OS was 24.7 months for the TUKYSA® combination group versus 19.2 months in the placebo combination group (HR for death=0.73; P=0.004). The estimated OS rate at 2 years was 51% in the TUKYSA® combination group and 40% in the placebo combination group. The OS benefit with the TUKYSA® combination was noted across all prespecified subgroups in the overall study population and was consistent with the primary analysis. The median duration of PFS was 7.6 months for the TUKYSA® combination group versus 4.9 months for the placebo combination group (HR for progression or death=0.57; P<0.00001), and PFS at 1 year was 29% and 14%, respectively.
Systemic treatment with TUKYSA® in combination with HERCEPTIN® and XELODA® provided consistent clinical benefit to patients with and without brain metastases. TUKYSA® combination doubled the intracranial Objective Response Rate, reduced the risk of intracranial progression or death by two-thirds in all patients with brain metastases. In this study population, the estimated 1-year intracranial PFS was 40% in the TUKYSA® group and 0% in the control group. In patients with untreated or treated and progressing (active) brain metastases, the estimated 1-year intracranial PFS was 35% in the TUKYSA® group, 0% in the control group, and in patients with treated (stable) brain metastases, was 53% in the TUKYSA® group and 0% in the control group. The TUKYSA® combination was well tolerated with a low rate of discontinuation due to toxicities. Common adverse events in the TUKYSA® group included diarrhea, Palmar-Plantar Erythrodysesthesia syndrome, nausea, vomiting and fatigue. Diarrhea and abnormal liver function tests were more common in the TUKYSA® group than in the control group.
It was concluded that with additional follow up, TUKYSA® in combination with HERCEPTIN® and XELODA® provided a clinically meaningful survival benefit, including those with brain metastases, supporting the use of this combination in patients with previously treated HER2-positive metastatic breast cancer, after progression on two HER2-targeted therapies.
Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Curigliano G, Mueller V, Borges V, et al. Ann Oncol. 2022;33:321-329.
VERZENIO® (Abemaciclib)
The FDA on October 12, 2021, approved VERZENIO® (Abemaciclib) with endocrine therapy (Tamoxifen or an Aromatase Inhibitor) for adjuvant treatment of adult patients with Hormone Receptor (HR)-positive, Human Epidermal growth factor Receptor 2 (HER2)-negative, node-positive, early breast cancer at high risk of recurrence and a Ki-67 score ≥20%, as determined by an FDA approved test. This is the first CDK 4/6 inhibitor approved for adjuvant treatment of breast cancer. VERZENIO® is a product of Eli Lilly and Company.
FDA also approved the Ki-67 IHC MIB-1 pharmDx (Dako Omnis) assay, submitted by Agilent, Inc., as a companion diagnostic for selecting patients for this indication.
KISQALI® Plus FEMARA® Improves Overall Survival in Advanced Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence. Approximately 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors and these patients are often treated with anti-estrogen therapy as first line treatment. However, resistance to hormonal therapy occurs in a majority of the patients.
Cyclin Dependent Kinases (CDK) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. Cyclin Dependent Kinases 4 and 6 (CDK4 and CDK6) phosphorylate RetinoBlastoma protein (RB), and initiate transition from the G1 phase to the S phase of the cell cycle. RetinoBlastoma protein has antiproliferative and tumor-suppressor activity and phosphorylation of RB protein nullifies its beneficial activities. CDK4 and CDK6 are activated in hormone receptor positive breast cancer, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation and associated with increased expression of CDK4. The understanding of the role of Cyclin Dependent Kinases in the cell cycle, has paved the way for the development of CDK inhibitors.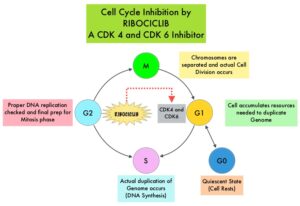
KISQALI® (Ribociclib) is an orally bioavailable, selective, small-molecule inhibitor of CDK4/6, preferentially inhibiting CDK4, that blocks the phosphorylation of RetinoBlastoma protein, thereby preventing cell-cycle progression and inducing G1 phase arrest. In a phase 1b study involving postmenopausal women with ER positive, HER2-negative advanced breast cancer, KISQALI® in combination with FEMARA® (Letrozole) demonstrated an Overall Response Rate (ORR) of 46% and a Clinical Benefit Rate of 79%, in treatment-naïve patients with advanced breast cancer. This led to the design of MONALEESA-2 trial.
MONALEESA-2 trial is a randomized, double-blind, placebo-controlled, Phase III study in which 668 patients were randomly assigned in a 1:1 ratio to receive either KISQALI® plus FEMARA® or placebo plus FEMARA®. Eligible patients included post-menopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer who had received no prior therapy for advanced disease. Treatment consisted of oral KISQALI® 600 mg daily on a 3-weeks on and 1-week off schedule, in 28-day treatment cycles plus FEMARA® 2.5 mg orally daily on a continuous schedule or placebo plus FEMARA®. Patients were stratified according to the presence or absence of liver or lung metastases and treatment was continued until disease progression or unacceptable toxicity. No treatment crossover was allowed. The median age was 62 years, close to 60% of the patients had visceral metastases, and patients were stratified according to the presence or absence of liver or lung metastases. The Primary end point was Progression Free Survival (PFS) and Secondary end points included Overall Survival (OS), Overall Response Rate (ORR), Clinical Benefit Rate (Overall Response plus Stable disease lasting 24 weeks or more), Safety, and Quality of Life assessments.
In the primary and updated analyses of the MONALEESA-2 trial, PFS was significantly longer with KISQALI® plus FEMARA® than with placebo plus FEMARA® (25.3 months versus 16.0 months; HR for disease progression or death=0.57; P<0.001). The Overall Survival data were immature at the time of the primary and updated analyses. The authors have now reported the findings from the protocol-specified final analysis of Overall Survival, which is a key Secondary end point.
After a median follow up of 6.6 years, a significant Overall Survival benefit was observed with KISQALI® plus FEMARA®, compared to placebo plus FEMARA®. The median Overall Survival was 63.9 months with KISQALI® plus FEMARA® and 51.4 months with placebo plus FEMARA® (HR=0.76; two-sided P=0.008). This Overall Survival benefit was consistent across all prespecified subgroups. The median time to first subsequent chemotherapy was 50.6 months in the KISQALI® group and 38.9 months in the placebo group (HR for receipt of first chemotherapy=0.74). No new safety signals were observed.
It was concluded from the analysis of the MONALEESA-2 trial that first line therapy with KISQALI® plus FEMARA® showed a significant Overall Survival benefit as compared with placebo plus FEMARA®, in patients with HR-positive, HER2-negative advanced breast cancer, with a 24% relative reduction in the risk of death. The authors added that MONALEESA trials of KISQALI® have shown a consistent Overall Survival benefit regardless of accompanying endocrine therapy, line of therapy, or menopausal status.
Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. Hortobagyi GN, Stemmer SM, Burris HA, et al. N Engl J Med 2022; 386:942-950
Overall Survival Benefit with the Addition of Capecitabine to Adjuvant Chemotherapy
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence.
Patients with early stage breast cancer often receive adjuvant chemotherapy to improve Overall Survival (OS), and this is even more so true for HER positive and triple negative (ER, PR and HER negative) breast cancer patients, who are at an increased risk to develop recurrent disease. Meta-analyses conducted by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) has shown a 20-25% relative risk reduction in breast cancer mortality with first-generation adjuvant chemotherapy regimens such as CMF (Cyclophosphamide/Methotrexate/Fluorouracil) and additional survival benefit with the Anthracyclines and Taxane based regimens. This benefit is dependent on the type of chemotherapy administered and chemotherapy dose intensity.
XELODA® (Capecitabine) is an oral prodrug of fluorouracil which is presently approved for the treatment of advanced breast cancer, but NOT for neoadjuvant or adjuvant treatment of early breast cancer. Meta-analysis of randomized trials has found that addition of Capecitabine to standard adjuvant chemotherapy regimens prolongs Disease Free Survival (DFS), whereas replacing a standard agent with Capecitabine did not improve DFS. Preclinical models have suggested that chemotherapy agents such as taxanes, and Cyclophosphamide increase thymidine phosphorylase concentration in the cancer cell, potentially leading to improved conversion of Capecitabine to fluorouracil within the tumor, suggesting that concomitant administration of Capecitabine with these agents improves efficacy, compared with single-agent Capecitabine. The researchers in this publication addressed the question whether addition of Capecitabine to these regimens could lead to improved survival outcomes.
The Finland Capecitabine Trial (FinXX) is a randomized, open-label, multicenter, Phase III trial that evaluated the addition of Capecitabine to an adjuvant chemotherapy regimen containing a taxane and an anthracycline for the treatment of early breast cancer. In this study, 1,500 patients with axillary node-positive or high-risk node-negative early breast cancer were randomly assigned to 6 cycles of either the Capecitabine arm- TX-CEX (N=753) or to the control group-T-CEF (N=747). TX-CEF consisted of Docetaxel 60 mg/m2 IV day 1 and Capecitabine 900 mg/m2 PO BID on days 1-15 of a 21-day cycle for 3 cycles followed by CEX consisting of Cyclophosphamide 600 mg/m2 IV Day 1, Epirubicin 75 mg/m2 IV on Day 1 and Capecitabine 900 mg/m2 PO BID on days 1-15 of a 21 day cycle for 3 cycles. T-CEF consisted of Docetaxel 80 mg/m2 IV Day 1, every 3 weeks for 3 cycles followed by CEF consisting of Cyclophosphamide 600 mg/m2 IV, Epirubicin 75 mg/m2 IV and Fluorouracil 600 mg/m2 IV, all administered on Day 1, every 3 weeks for 3 cycles. Adjuvant endocrine therapy was initiated within 2 months after completion of chemotherapy if the tumor was ER or PR-positive. Radiotherapy was given after completion of chemotherapy according to each institution’s practice. Adjuvant Trastuzumab was approved while the trial accrual was ongoing and was allowed for women with HER2-positive cancer after May 2005, and adjuvant Trastuzumab was administered to 13% patients assigned to TX-CEF and 11% patients assigned to T-CEF. The median patient age was 52.5 yrs, 76% of patients had ER-positive tumors, 19% had HER2-positive cancer, 13% had Triple Negative Breast Cancer, more than 90% had T1 or T2 tumors, and 89% were node positive. The researchers then performed a protocol-scheduled analysis of Overall Survival on the basis of approximately 15-year follow up of the patients.
At a median follow up of 15.3 years, the Overall Survival was 77.6% in the TX-CEX group and 73.3% in the T-CEF group (HR=0.81; P=0.037). Exploratory subgroup analysis suggested that patients with ER-negative disease and those with Triple Negative Breast Cancer lived longer with the addition of Capecitabine (TX-CEX regimen), than those treated with T-CEF.
It was concluded that the addition of Capecitabine to a chemotherapy regimen significantly improved Overall Survival at median follow up of 15 years in a patient population with early breast cancer, suggesting that Capecitabine may be an important addition to adjuvant chemotherapy in patients with high risk disease.
Adjuvant Capecitabine for Early Breast Cancer: 15-Year Overall Survival Results from a Randomized Trial. Joensuu H, Kellokumpu-Lehtinen P-L , Huovinen R, et al. DOI: 10.1200/JCO.21.02054 Journal of Clinical Oncology. Published online January 12, 2022.
Adjuvant VERZENIO® in High Risk Early Stage Breast Cancer: Updated Efficacy and Ki-67 Analysis
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence. About 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors, and Hormone Receptor (HR)-positive/HER2-negative breast cancer is the most frequently diagnosed molecular subtype. Majority of these patients are diagnosed with early stage disease and are often cured with a combination of surgery, radiotherapy, chemotherapy, and hormone therapy. However approximately 20% of patients will experience local recurrence or distant relapse during the first 10 years of treatment. This may be more relevant for those with high risk disease, among whom the risk of recurrence is even greater during the first 2 years while on adjuvant endocrine therapy, due to primary endocrine resistance. More than 75% of the early recurrences are seen at distant sites.
Cyclin Dependent Kinases (CDKs) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. CDK 4 and 6 phosphorylate RetinoBlastoma protein (RB), and initiate transition from the G1 phase to the S phase of the cell cycle. RetinoBlastoma protein has antiproliferative and tumor-suppressor activity. Phosphorylation of RB protein nullifies its beneficial activities. CDK4 and CDK6 are activated in HR-positive breast cancer, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation. The understanding of the role of CDKs in the cell cycle, has paved the way for the development of CDK inhibitors.
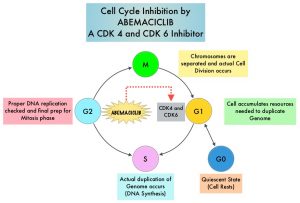 VERZENIO® (Abemaciclib) is an oral, selective inhibitor of CDK4 and CDK6 kinase activity, and prevents the phosphorylation and subsequent inactivation of the Rb tumor suppressor protein, thereby inducing G1 cell cycle arrest and inhibition of cell proliferation. VERZENIO® is structurally distinct from other CDK 4 and 6 inhibitors (such as Ribociclib and Palbociclib) and is 14 times more potent against Cyclin D1/CDK 4 and Cyclin D3/CDK 6, in enzymatic assays, but potentially less toxic than earlier pan-CDK inhibitors. At higher doses, only VERZENIO® causes significant cancer cell death, compared with other CDK4/6 inhibitors, suggesting that this drug may be affecting proteins, other than CDK4/6. Additionally, preclinical studies have demonstrated that VERZENIO® may have additional therapeutic benefits for a subset of tumors that are unresponsive to treatment or have grown resistant to other CDK4/6 inhibitors. It has also been shown to cross the blood-brain barrier.
VERZENIO® (Abemaciclib) is an oral, selective inhibitor of CDK4 and CDK6 kinase activity, and prevents the phosphorylation and subsequent inactivation of the Rb tumor suppressor protein, thereby inducing G1 cell cycle arrest and inhibition of cell proliferation. VERZENIO® is structurally distinct from other CDK 4 and 6 inhibitors (such as Ribociclib and Palbociclib) and is 14 times more potent against Cyclin D1/CDK 4 and Cyclin D3/CDK 6, in enzymatic assays, but potentially less toxic than earlier pan-CDK inhibitors. At higher doses, only VERZENIO® causes significant cancer cell death, compared with other CDK4/6 inhibitors, suggesting that this drug may be affecting proteins, other than CDK4/6. Additionally, preclinical studies have demonstrated that VERZENIO® may have additional therapeutic benefits for a subset of tumors that are unresponsive to treatment or have grown resistant to other CDK4/6 inhibitors. It has also been shown to cross the blood-brain barrier.
VERZENIO® is presently approved by the FDA as monotherapy as well as in combination with endocrine therapy for patients with HR-positive, HER2- negative advanced breast cancer. The addition of VERZENIO® to FASLODEX® (Fulvestrant) resulted in a statistically significant improvement in Overall Survival (OS) among patients with HR-positive, HER2-negative advanced breast cancer, who had progressed on prior endocrine therapy. The goal of monarchE was to evaluate the additional benefit of adding a CDK4/6 inhibitor to endocrine therapy in the adjuvant setting, for patients with HR-positive, HER2-negative, high risk, early breast cancer.
The International monarchE trial, is an open-label, randomized, Phase III study, which included 5637 patients, who were pre- and postmenopausal, with HR-positive, HER2-negative early breast cancer, and with clinical and/or pathologic risk factors that rendered them at high risk for relapse. The researchers defined high risk as the presence of four or more positive axillary lymph nodes, or 1-3 three positive axillary lymph nodes, with either a tumor size of 5 cm or more, histologic Grade 3, or centrally tested high proliferation rate (Ki-67 of 20% or more). Following completion of primary therapy which included both adjuvant and neoadjuvant chemotherapy and radiotherapy, patients were randomly assigned (1:1) to VERZENIO® 150 mg orally twice daily for 2 years plus 5-10 years of physicians choice of endocrine therapy as clinically indicated (N=2808), or endocrine therapy alone (N=2829). The median patient age was 51 years, about 43% of the patients were premenopausal, and 95% of patients had prior chemotherapy. Approximately 60% of patients had 4 or more positive lymph nodes. The Primary endpoint was Invasive Disease Free Survival (IDFS), and Secondary end points included Distant Relapse Free Survival (DRFS), Overall Survival (OS), and Safety. The researchers provided updated results from the prespecified Primary outcome analysis, additional follow-up analysis conducted at regulatory request, as well as outcomes from prespecified subpopulations, based on Ki-67 levels.
At the time of Primary outcome analysis, with a median follow up of 19 months, 1,437 patients (25.5%) had completed the 2 year treatment period and 3,281 patients (58.2%) were in the 2 year treatment period. The combination of VERZENIO® plus endocrine therapy demonstrated superior Invasive Disease Free Survival (IDFS) compared to endocrine therapy alone, with a 29% reduction in the risk of developing invasive disease (P=0.0009; HR=0.71). The 2-year IDFS in the combination group was 92.3% and 89.3% in the endocrine therapy alone treatment group, with an absolute improvement of 3.0%. Further, there was an improvement in the 2-year distant Relapse Free Survival (DRFS) rate among patients who received the combination treatment compared with those who received endocrine therapy alone, corresponding to an absolute difference of 3.0% at 2 years (93.8% versus 90.8%, respectively; HR=0.69; P<0.001).
With 8 months of additional follow up, at a median of 27 months and with 90% of patients off treatment, the benefit with the combination of VERZENIO® plus endocrine therapy was maintained for IDFS (HR=0.70; P<0.0001) and DRFS (HR=0.69; P<0.0001), demonstrating a 30% risk reduction for IDFS and 31% risk reduction for DRFS. There was continued treatment benefit over time that extended beyond the 2-year treatment period of VERZENIO®. With more patients at risk for recurrence at 3 years, the data demonstrated absolute improvements in 3-year IDFS and DRFS rates of 5.4% and 4.2%, respectively. This treatment benefit in IDFS and DRFS was noted across prespecified subgroups. Further, the benefit with VERZENIO® was consistent, regardless of Ki-67 index. Overall Survival data was immature at the time of this analysis.
It was concluded that adjuvant VERZENIO® combined with endocrine therapy continued to demonstrate statistically significant and clinically meaningful improvement in Invasive Disease Free Survival and Distant Relapse Free Survival, among patients with HR-positive, HER2-negative, node-positive, high risk, early breast cancer, regardless of Ki-67 status.
Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Harbeck N, Rastogi P, Martin M, et al. Annals of Oncology 2021;32: 1457-1459.
Elacestrant in Metastatic Breast Cancer Progressing on CDK4/6 Therapy and ESR1-Mutant Subtype
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 290,560 new cases of breast cancer will be diagnosed in 2022 and about 43,780 individuals will die of the disease, largely due to metastatic recurrence. Approximately 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors. The most common subtype of metastatic breast cancer is Hormone Receptor-positive (HR-positive), HER2-negative breast cancer (65% of all metastatic breast tumors), and these patients are often treated with anti-estrogen therapy as first line treatment. However, resistance to hormonal therapy occurs in a majority of the patients, with a median Overall Survival (OS) of 36 months. With the development of Cyclin Dependent Kinases (CDK) 4/6 inhibitors, endocrine therapy plus a CDK4/6 inhibitor is the mainstay for the management of ER+/HER2- metastatic breast cancer as first-line therapy. Even with this therapeutic combination, most patients will eventually experience disease progression, including development of ESR1 (Estrogen Receptor gene alpha) mutations.
ESR1 is the most common acquired mutation noted in breast tumors as they progress from primary to metastatic setting. These mutations promote ligand independent Estrogen Receptor activation and have been shown to promote resistance to estrogen deprivation therapy. It appears that ESR1 mutations are harbored in metastatic ER+ breast cancers with prior Aromatase Inhibitor (AI) therapy, but not in primary breast cancers, suggesting that ESR1 mutations may be selected by prior therapy with an AI, in advanced breast cancer. In a recently published study (JAMA Oncol.2016;2:1310-1315), ESR1 mutations Y537S and D538G mutations detected in baseline plasma samples from ER+/HER- advanced breast cancer patients, was associated with shorter Overall Survival. In this study it was noted that there was a three-fold increase in the prevalence of these mutations in patients who had failed first line hormonal therapy for metastatic disease, compared with those who were initiating first line therapy for advanced breast cancer (33% versus 11%).
Fulvestrant is a parenteral, Selective Estrogen Receptor Degrader (SERD) and is the only SERD approved for the treatment of postmenopausal women with HR-positive metastatic breast cancer. However, acquired ESR1 mutations can also occur following Fulvestrant treatment, possibly because of poor bioavailability and incomplete ER blockade when administered intramuscularly. There is therefore an urgent unmet need for an alternate SERD that has activity in tumors harboring ESR1 mutations, and has improved bioavailability allowing oral administration.
Elacestrant is an oral, nonsteroidal, Selective Estrogen Receptor Degrader (SERD) that degrades the Estrogen Receptor (ER) in a dose-dependent manner and inhibits estradiol-dependent functions of ER target gene transcription induction and breast cancer cell proliferation. Estradiol-stimulated tumor growth was diminished by Elacestrant in the ER+ xenograft models derived from heavily pretreated patients, including models resistant to CDK 4/6 inhibitors, Fulvestrant and those harboring ESR1 mutations Y537S and D538G. In an early Phase I trial, Elacestrant was noted to have an acceptable safety profile, and demonstrated single-agent activity with confirmed Partial Responses in heavily pretreated patients with ER+ metastatic breast cancer.
EMERALD trial is a multicenter, International, randomized, open-label, Phase III study designed to evaluate the benefit of Elacestrant in patients with ER+ HER2- advanced or metastatic breast cancer. In this study, 477 postmenopausal women with ER+/HER2- metastatic breast cancer were randomly assigned 1:1 to receive either Elacestrant 400 mg orally daily (N=239) or the Standard of Care which included investigator’s choice of Fulvestrant or an Aromatase Inhibitor including Anastrozole, Letrozole, or Exemestane (N=238). Treatment was given until disease progression. Both treatment groups were well balanced. The median patient age was 63 years, and patients must have progressed or relapsed on or after 1 or 2 lines of endocrine therapy for advanced disease, one of which was given in combination with a CDK4/6 inhibitor, had 1 or fewer lines of chemotherapy for advanced disease, and had an ECOG performance status of 0 or 1. In the study, 48% had tumors with mutated ESR1 and these patients were evenly distributed in both treatment groups. Patients were stratified by ESR1-mutation status, prior treatment with Fulvestrant, and visceral metastases. The co-Primary end points were Progression Free Survival (PFS) in the overall population, and in those with ESR1 mutations. Overall Survival (OS) was a Secondary end point.
Treatment with Elacestrant resulted in a statistically significant and clinically meaningful improvement in PFS, compared with Standard of Care. There was a 31% reduction in the risk of progression or death in the Elacestrant group for all patients (HR=0.69; P=0.0018) and a 45% reduction in the risk of progression or death among those with ESR1 mutations (HR=0.55; P=0.0005).
The PFS at 12 months with Elacestrant was 22.3% in all patients compared with 9.4% for those receiving the Standard of Care treatment. Among the ESR1 mutation group, the 12 month PFS rate was more pronounced and was 26.8% with Elacestrant, compared to 8.2% with Standard of Care. The benefits with Elacestrant compared with Standard of Care, was consistent across multiple prespecified subgroups including patients who had received prior Fulvestrant. There also was a trend toward improved Overall Survival in patients who received Elacestrant, compared with Standard of Care. The final OS results however are not expected until late 2022. Elacestrant was well tolerated and treatment discontinuation rate was not significantly different between the two treatment groups.
It was concluded that Elacestrant is the first oral Selective Estrogen Receptor Degrader that demonstrated significant and clinically meaningful improvement in PFS compared with Standard of Care endocrine therapy in patients with ER+/ HER2- metastatic breast cancer in the second/third line after treatment with a CDK4/6 inhibitor, and has the potential to become the new standard of care in the study population.
Elacestrant, an oral selective estrogen receptor degrader (SERD), vs investigator’s choice of endocrine monotherapy for ER+/HER2- advanced/metastatic breast cancer (mBC) following progression on prior endocrine and CDK4/6 inhibitor therapy: Results of the EMERALD phase 3 trial. Bardia A, Neven P, Streich G, et al. Presented at 2021 San Antonio Breast Cancer Symposium; December 7-10, 2021; San Antonio, TX. Abstract GS2-02.
Defining Patient Groups With Triple Negative Breast Cancer Who Derive Benefit From KEYTRUDA® plus Chemotherapy
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 284,200 new cases of breast cancer will be diagnosed in 2021 and about 44,130 individuals will die of the disease, largely due to metastatic recurrence. Triple Negative Breast Cancer (TNBC) is a heterogeneous, molecularly diverse group of breast cancers and are ER (Estrogen Receptor), PR (Progesterone Receptor) and HER2 (Human Epidermal Growth Factor Receptor-2) negative. TNBC accounts for 15-20% of invasive breast cancers, with a higher incidence noted in young patients. It is usually aggressive, and tumors tend to be high grade and patients with TNBC are at a higher risk of both local and distant recurrence. Those with metastatic disease have one of the worst prognoses of all cancers with a median Overall Survival of 13 months. The majority of patients with TNBC who develop metastatic disease do so within the first 3 years after diagnosis, whereas those without recurrence during this period of time have survival rates similar to those with ER-positive breast cancers.
The lack of known recurrent oncogenic drivers in patients with metastatic TNBC, presents a major therapeutic challenge. It appears that there are subsets of patients with TNBC who may be inherently insensitive to cytotoxic chemotherapy. Three treatment approaches appear to be promising and they include immune therapies, PARP inhibition, and inhibition of PI3K pathway. Previously published studies have shown that presence of tumor-infiltrating lymphocytes was associated with clinical benefit, when treated with chemotherapy and immunotherapy, in patients with TNBC, and improved clinical benefit was observed in patients with immune-enriched molecular subtypes of metastatic TNBC.
KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. It thereby reverses the PD-1 pathway-mediated inhibition of the immune response, and unleashes the tumor-specific effector T cells. The rationale for combining chemotherapy with immunotherapy is that cytotoxic chemotherapy releases tumor-specific antigens, and immune checkpoint inhibitors such as KEYTRUDA® when given along with chemotherapy can enhance endogenous anticancer immunity. Single agent KEYTRUDA® in metastatic TNBC demonstrated durable antitumor activity in several studies, with Objective Response Rates (ORRs) ranging from 10-21% and improved clinical responses in patients with higher PD-L1 expression. When given along with chemotherapy as a neoadjuvant treatment for patients with high-risk, early-stage TNBC, KEYTRUDA® combination achieved Pathological Complete Response rate of 65%, regardless of PD-L1 expression. Based on this data, KEYTRUDA® in combination with chemotherapy was studied, for first line treatment of advanced TNBC.
KEYNOTE-355 is a randomized, double-blind, Phase III study, which evaluated the benefit of KEYTRUDA® in combination with one of the three different chemotherapy regimens, nab-Paclitaxel, Paclitaxel, or the non-taxane containing Gemzar/Carboplatin, versus placebo plus one of the three chemotherapy regimens, in patients with previously untreated or locally recurrent inoperable metastatic TNBC. In this study, 847 patients were randomized 2:1 to receive either KEYTRUDA® 200 mg IV on day 1 of each 21-day cycle along with either nab-Paclitaxel 100 mg/m2 IV on days 1, 8 and 15 of each 28-day cycle, Paclitaxel 90 mg/m2 IV on days 1, 8 and 15 of each 28-day cycle, or Gemcitabine 1000 mg/m2 IV plus Carboplatin AUC 2, IV on days 1 and 8 of each 21-day cycle (N= 566) or placebo along with one of the three chemotherapy regimens (N= 281). This study was not designed to compare the efficacy of the different chemotherapy regimens. Treatment was continued until disease progression. Patients were stratified by chemotherapy, PD-L1 tumor expression (CPS-Combined Positive Score of 1 or higher versus CPS of less than 1), and prior treatment with the same class of neoadjuvant/adjuvant chemotherapy (Yes versus No). The baseline characteristics of treatment groups were well-balanced. The co-Primary end points of the trial were Progression Free Survival (PFS) and Overall Survival (OS) in patients with PD-L1-positive tumors, and in all patients. Secondary end points were Objective Response Rate (ORR), Duration of Response, Disease Control Rate, and Safety.
In the primary analysis of the KEYNOTE-355 trial, the Overall Survival results after a median follow up of 44.1 months in the subgroup of patients with PD-L1 CPS (Combined Positive Score) of 10 or more was significantly better with first line KEYTRUDA® plus chemotherapy versus placebo plus chemotherapy (23.0 months versus 16.1 months, respectively; HR=0.73; P=0.0093). This represented a 27% reduction in the risk of death with the KEYTRUDA® combination. KEYTRUDA® in combination with chemotherapy, also significantly improved PFS in patients with CPS (Combined Positive Score) of 10 or greater. The median PFS was 9.7 months for KEYTRUDA® plus chemotherapy, compared with 5.6 months for placebo plus chemotherapy (HR=0.65, P=0.0012). This represented a 35% reduction in the risk of disease progression. However, among patients with CPS of 1 or greater, the median PFS was not considered statistically significant, based on prespecified statistical criteria.
The researchers here in presented the results of a subgroup analysis, stratified by levels of PD-L1 expression, as assessed by CPS score. In the subgroups with CPS scores of less than 1 and 1-9, Overall Survival was similar for KEYTRUDA® plus chemotherapy and placebo plus chemotherapy. However, in subgroups with CPS 10-19 and CPS 20 or more, there was sustained separation of the Overall survival curves starting at approximately 10 months and the survival was improved by about 28%.
The authors noted that the general trend for PFS was consistent with that observed for Overall Survival, with improving PFS trend among those subgroups with PD-L1 enriched CPS of 10 or more. In the subgroup of patients with a CPS of 10-19 and CPS of 20 or more, the addition of KEYTRUDA® to chemotherapy resulted in a more sustained separation of PFS curves, beginning at approximately 4 months, compared with placebo plus chemotherapy. The Hazard Ratios for these two groups were 0.70 and 0.62, respectively. Toxicities of any grade were reported in 96% of the experimental group and 95% of the placebo plus chemotherapy group. The rate of Grades 3-5 treatment-related adverse events was 68.1% and 66.9%, respectively and the majority of treatment discontinuations in this study were for progressive disease.
The researchers based on this subgroup analyses concluded that a CPS of 10 or more is a reasonable cutoff to define the population of women with metastatic Triple Negative Breast Cancer, expected to derive treatment benefit from KEYTRUDA® plus chemotherapy, lending further support to KEYTRUDA® plus chemotherapy as a standard of care treatment regimen for this group of patients.
Final results of KEYNOTE-355: randomized, double-blind, phase 3 study of pembrolizumab + chemotherapy vs placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. Cortés J, Cescon DW, Rugo HS, et al. Presented at: 2021 San Antonio Breast Cancer Symposium; December 7-10, 2021; San Antonio, TX. Abstract GS1-02.
Postmenopausal Women with Node Positive Breast Cancer May Not Benefit From Chemotherapy
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 284,200 new cases of breast cancer will be diagnosed in 2021 and about 44,130 individuals will die of the disease, largely due to metastatic recurrence. Approximately 25% of patients with Hormone Receptor (HR)-positive, HER2-negative early breast cancer have metastatic lymph node involvement and two third of these patients are postmenopausal. Majority of these patients currently receive adjuvant chemotherapy.
The Oncotype DX breast cancer assay, is a multigene genomic test that analyzes the activity of a group of 21 genes and is able to predict the risk of breast cancer recurrence and likelihood of benefit from systemic chemotherapy, following surgery, in women with early stage breast cancer. Chemotherapy recommendations for early stage, HR-positive, HER-negative, early stage breast cancer patients, are often made based on tumor size, grade, ImmunoHistoChemical (IHC) markers such as Ki-67, nodal status and Oncotype DX Recurrence Score (RS) assay.
In the ground-breaking TAILORx (Trial Assigning Individualized Options for Treatment) study which enrolled 10,273 patients with HR-positive, HER2-negative, axillary node-negative breast cancer, patients were divided into three groups based on their Recurrence Score. Patient with Intermediate Recurrence Score of 11-25 were randomly assigned to receive endocrine therapy alone or endocrine therapy and adjuvant chemotherapy. There was no benefit noted from adding chemotherapy to endocrine therapy, for women older than 50 years in this Intermediate RS group, suggesting that a significant percentage of women with node-negative breast cancer do not achieve substantial benefit from chemotherapy. For women 50 years old or younger who received chemotherapy and had a Recurrence Score of at least 16, there was a lower rate of distant recurrence, and the absolute benefit increased with increasing recurrence score. Further, the risk of recurrence and benefit of chemotherapy was further influenced by the tumor size and grade.
Whether the results of TAILORx can be extrapolated to women with node-positive breast cancer has remained unclear. It is estimated that approximately 85% of women with node-positive disease have Recurrence Score results of 0-25. The RxPONDER (A Clinical Trial RX for Positive Node, Endocrine Responsive Breast Cancer) trial was designed to determine the benefit of chemotherapy, in patients with HR-positive, HER2-negative breast cancer and 1-3 positive axillary lymph nodes (nodal stage N1), who had a Recurrence Score of 0-25. This trial did not include pre and postmenopausal women with Recurrence Score results 26-100, based on previously published studies suggesting that this patient group benefited from chemotherapy.
SWOG S1007 (RxPONDER) is an multicenter, international, prospective, randomized, Phase III trial, in which patients with HR-positive, HER2-negative breast cancer with 1-3 positive axillary lymph nodes were included, to determine which patients would benefit from chemotherapy and which patients could safely avoid it. In this study, a total of 5083 HR-positive, HER2-negative breast cancer patients with 1-3 positive lymph nodes and Oncotype DX Recurrence Score of less than 25 were randomly assigned 1:1 to receive chemotherapy plus endocrine therapy (N=2547) or endocrine therapy alone (N=2536). The median patient age was 57.5 years and approximately two-thirds of patients were postmenopausal and one-third were premenopausal and had no contraindications to taxane and/or anthracycline based chemotherapy. Patients were stratified by Recurrence Score (0-13 versus 14-25), menopausal status, and axillary nodal dissection versus sentinel node biopsy. The Primary endpoint was Invasive Disease Free Survival (IDFS), defined as local, regional, or distant recurrence, any second invasive cancer, or death from any cause, and whether the effect depended on the Recurrence Score. Secondary endpoints included distant Relapse Free Survival (RFS) and Overall Survival (OS).
At a median follow up of 6.1 years, the chemotherapy benefit with respect to increasing invasive DFS differed according to menopausal status. Among postmenopausal women, in this updated analysis with longer follow up, the invasive DFS at 5 years was 91.9% in the endocrine therapy alone group, and was 91.3% in those treated with chemotherapy plus endocrine therapy (HR=1.02; P=0.89). Postmenopausal women with recurrence scores of 0 to 25 continued to NOT benefit from adjuvant chemotherapy.
Among premenopausal women however, the invasive DFS at 5 years was 89% in the endocrine therapy alone group and 93.9% % in those treated with chemotherapy plus endocrine therapy (HR=0.64; P=0.004). There was a 5-year absolute benefit of 4.9% for invasive DFS with chemotherapy among premenopausal women. There was a similar increase noted in the distant Relapse Free Survival (HR=0.58; P=0.009). The relative chemotherapy benefit did not increase as the Recurrence Score increased.
It was concluded from this practice-changing study that postmenopausal women with HR-positive, HER2-negative breast cancer with 1-3 positive nodes and Oncotype DX Recurrence Score of 25 or less, can safely avoid receiving adjuvant chemotherapy, whereas premenopausal patients with 1-3 positive nodes and a Recurrence Score of 25 or less benefited from chemotherapy plus endocrine therapy and had a longer invasive Disease Free Survival and distant Relapse Free Survival, than those who received endocrine therapy alone.
21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. Kalinsky K, Barlow WE, Gralow JR, et al. N Engl J Med 2021;385:2336-2347
Risk of Cardiovascular Diseases among Older Breast Cancer Survivors
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 284,200 new cases of breast cancer will be diagnosed in 2021 and about 44,130 individuals will die of the disease, largely due to metastatic recurrence.
Significant progress in breast cancer screening techniques, as well as new and novel therapies, have resulted in early cancer detection and improvement in the breast cancer 5-year survival rate in the US from 75% in the 1970s to 91% in the 2010s. Cardiovascular Disease (CVD) is the most frequent cause of noncancer-related death, and cardiotoxicities associated with cancer treatments may increase cardiovascular risk in this population of breast cancer survivors. However, few studies have in detail quantified the risks of the different clinically important cardiovascular outcomes. The authors therefore assessed the prevalence of the different clinically specific cardiovascular outcomes at breast cancer diagnosis, and their incidence after diagnosis, among survivors 65 years or older in the US, and compared this with similar women without cancer.
The researchers performed a matched cohort study using prospectively collected data from the SEER-Medicare linked claims-based database and identified all women older than 65 years of age with an incident Stage I-III breast cancer diagnosis in 2004 through 2013. Each patient with breast cancer was matched at diagnosis with 5 cancer-free female counterparts. Baseline prevalence of specific cardiovascular outcomes was measured, and the risk for individual cardiovascular outcomes during follow up was calculated, taking into consideration time since diagnosis, race/ethnicity, prior Cardiovascular Disease (CVD), and age. This study included a total of 91,473 women with breast cancer and 454,197 without breast cancer.
It was noted that women with breast cancer had lower baseline prevalence of all CVDs. Breast cancer survivors had substantially increased risks of Deep Vein Thrombosis and pericarditis, compared with cancer-free female counterparts. There was also evidence of smaller increased risks of sudden cardiac arrest, arrhythmia, heart failure, and valvular heart disease. The increased risks of arrhythmia, heart failure, pericarditis, and Deep Vein Thrombosis were most pronounced in the first year and persisted for more than 5 years after cancer diagnosis. There was evidence of a decreased risk of incident angina, myocardial infarction, revascularization, peripheral vascular disease, and stroke in breast cancer survivors, although this was not constant over time.
The CVD risk during follow up was consistently higher in African American women diagnosed with breast cancer compared with Caucasian women, regardless of whether there was an overall increased or decreased risk of outcomes during the entire follow up period, and this is consistent with racial differences in overall CVD risk in the US.
Finally, there was consistently a greater risk of all cardiovascular outcomes in those diagnosed with Stage III, Grade 3, and ER/PR-negative breast cancer, which may be a reflection of the more aggressive cancer treatment regimens used in these subtypes.
The authors concluded that there is evidence of increased risk of several cardiovascular diseases in elderly women diagnosed with breast cancer in the US, compared with similar women without cancer, with this risk persisting for several years after diagnosis. They added that these results highlight the importance of periodic cardiovascular evaluation throughout the long term follow up of women diagnosed with breast cancer.
Risk of Cardiovascular Diseases Among Older Breast Cancer Survivors in the United States: A Matched Cohort Study. Matthews AA, Hinton SP, Stanway S, et al. J Natl Compr Canc Netw 2021;19:275-284.
