The FDA on May 22, 2020 approved ALUNBRIG® for adult patients with Anaplastic Lymphoma Kinase (ALK)-positive metastatic Non-Small Cell Lung Cancer (NSCLC), as detected by an FDA-approved test. ALUNBRIG® is a product of ARIAD Pharmaceuticals Inc.
Tag: Lung Cancer: Non-Small Cell
FDA Approves ALUNBRIG® for First Line Treatment of ALK Positive Non Small Cell Lung Cancer
SUMMARY: The FDA on May 22, 2020 approved approved ALUNBRIG® (Brigatinib) for the first-line treatment of patients with ALK-positive metastatic Non Small Cell Lung Cancer (NSCLC), as detected by an FDA-approved test. Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2020, about 228, 820 new cases of lung cancer will be diagnosed and 135,720 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of NSCLC, 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large Cell Carcinomas. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, Adenocarcinoma now is the most frequent histologic subtype of lung cancer.
The discovery of rearrangements of the Anaplastic Lymphoma Kinase (ALK) gene in some patients with advanced NSCLC and adenocarcinoma histology, led to the development of agents such as XALKORI® (Crizotinib), ZYKADIA® (Ceritinib), ALECENSA® (Alectinib) and ALUNBRIG® (Brigatinib), with promising results. It has become clear that appropriate, molecularly targeted therapy for tumors with a molecular abnormality, results in the best outcomes. According to the US Lung Cancer Mutation Consortium (LCMC), two thirds of patients with advanced adenocarcinoma of the lung, have a molecular driver abnormality. The most common oncogenic drivers in patients with advanced adenocarcinoma of the lung are, KRAS in 25%, EGFR in 21% and ALK in 8% as well as other mutations in BRAF, HER2, AKT1 and fusions involving RET and ROS oncogenes. These mutations are mutually exclusive, and the presence of two simultaneous mutations, are rare.
The new approval for ALUNBRIG® was based on results from the Phase III ALTA 1L (ALK in Lung Cancer Trial of BrigAtinib in 1st Line) trial, which is a global, ongoing, randomized, open-label, comparative, multicenter study, in which investigators compared the efficacy and safety of ALUNBRIG® with XALKORI® (Crizotinib) in 275 patients with Stage IIIB/IV ALK positive, locally advanced or metastatic NSCLC, who have not received prior treatment with an ALK inhibitor, but may have received 1 prior regimen of chemotherapy or no chemotherapy in the advanced setting. Patients were randomized 1:1 to receive either ALUNBRIG® 180 mg orally once daily (N=137), with a 7-day lead-in period at 90 mg, or XALKORI® 250 mg orally twice daily (N=138). Crossover from the XALKORI® arm to receive ALUNBRIG® was permitted at BICR (Blinded Independent Review Committee)-assessed Progression Free Survival (PFS). The median age was 59 years, and 55% of patients were female. Twenty-nine percent had brain metastases at baseline with comparable pre-enrollment central nervous system (CNS) radiotherapy rates among both cohorts. Overall, 27% of patients had prior chemotherapy in the locally advanced or metastatic setting. The Primary endpoint was BIRC assessed PFS and Secondary endpoints included Objective Response Rate (ORR), Intracranial ORR, Intracranial PFS, Overall Survival (OS), safety, and tolerability.
At a median follow up of 25 months, it was noted that ALUNBRIG® reduced the risk of disease progression or death by 51% compared with XALKORI® (HR=0.49; P=0.0007), with a median PFS of 24 months as assessed by a BIRC versus 11 months for XALKORI®. The confirmed ORR as assessed by BIRC was 74% with ALUNBRIG® and 62% for XALKORI®. The median duration of response (DOR) was not reached, and 13.8 months with ALUNBRIG® and XALKORI®, respectively.
After more than two years of follow-up, ALUNBRIG® demonstrated superiority over XALKORI®, with significant anti-tumor activity observed, especially in patients with baseline brain metastases. The confirmed intracranial ORR for patients with measurable brain metastases at baseline, treated with ALUNBRIG® was 78% versus 26% for patients treated with XALKORI®. The median intracranial Duration of Response in confirmed responders with measurable brain metastases at baseline was Not Reached with ALUNBRIG® and 9.2 months with XALKORI®, respectively. The median intracranial PFS was 24 months with ALUNBRIG®, compared with 5.6 months for XALKORI®. ALUNBRIG® reduced the risk of intracranial disease progression or death by 69% in patients who had brain metastases at baseline (HR=0.31).
Additionally, patients in the ALUNBRIG® group also experienced significant improvements in Health-Related Quality of Life, with delay in the median time to worsening in Global Health Score by 27 months versus 8 months with XALKORI®, as well as delay in the time to worsening and prolonged duration of improvement in fatigue, nausea and vomiting, appetite loss, and emotional and social functioning. Further, the duration of improvement in QoL with ALUNBRIG® was Not Reached versus 12 months with XALKORI®.
It was concluded that ALUNBRIG® demonstrated a statistically and clinically significant improvement in Progression Free Survival when compared to XALKORI® in ALK inhibitor-naïve, ALK positive NSCLC, with superior efficacy especially among those with brain metastases at baseline.
Brigatinib vs crizotinib in patients with ALK inhibitor-naive advanced ALK+ NSCLC: Updated results from the phase III ALTA-1L trial. Camidge R, Kim HR, Ahn M, et al. Presented at the 2019 ESMO Asia Congress, November 23, 2019.
TECENTRIQ® (Atezolizumab)
The FDA on May 18, 2020, approved TECENTRIQ® for the first-line treatment of adult patients with metastatic Non-Small Cell Lung Cancer (NSCLC) whose tumors have high PD-L1 expression (PD-L1 stained 50% or more of tumor cells [TC 50% or more] or PD-L1 stained Tumor-Infiltrating Immune Cells [IC] covering 10% or more of the tumor area [IC 10% or more]), with no EGFR or ALK genomic tumor aberrations. TECENTRIQ® is a product of Genentech Inc.
OPVIDO® (Nivolumab) plus YERVOY® (Ipilimumab)
The FDA on May 15, 2020 approved the combination of OPDIVO® plus YERVOY® as first-line treatment for patients with metastatic Non-Small Cell Lung Cancer, whose tumors express PD-L1 (1% or more), as determined by an FDA-approved test, with no Epidermal Growth Factor Receptor (EGFR) or Anaplastic Lymphoma Kinase (ALK) genomic tumor aberrations. Both OPDIVO® and YERVOY® are products of Bristol-Myers Squibb Co.
RETEVMO® (Selpercatinib)
The FDA on May 8, 2020, granted accelerated approval to RETEVMO® for the following indications:
1) Adult patients with metastatic RET fusion-positive Non-Small Cell Lung Cancer (NSCLC).
2) Adult and pediatric patients 12 years of age or older with advanced or metastatic RET-mutant Medullary Thyroid Cancer (MTC) who require systemic therapy.
3) Adult and pediatric patients 12 years of age or older with advanced or metastatic RET fusion-positive thyroid cancer who require systemic therapy, and who are Radioactive Iodine-refractory (if Radioactive Iodine is appropriate).
RETEVMO® is a product of Eli Lilly and Company.
FDA Approves TABRECTA® for Metastatic Non-Small Cell Lung Cancer
SUMMARY: The FDA on May 6, 2020, granted accelerated approval to TABRECTA® (Capmatinib) for adult patients with metastatic Non-Small Cell Lung Cancer (NSCLC), whose tumors have a mutation that leads to Mesenchymal-Epithelial Transition (MET) exon 14 skipping, as detected by an FDA-approved test. The FDA also approved the FoundationOne CDx assay (Foundation Medicine, Inc.) as a companion diagnostic for TABRECTA®.
MET is a widely expressed Receptor Tyrosine Kinase and plays a pivotal role in cell growth, proliferation and survival. The MET gene encodes for a protein known as the Hepatocyte Growth Factor (HGF) Receptor. Upon binding by Hepatocyte Growth Factor (HGF), the HGF Receptor is activated, with resulting activation of the downstream RAS/RAF/MEK/ERK and PI3K/AKT/mTOR signaling pathways, thereby serving different important biological functions. Alterations in the MET gene leading to abnormal MET signaling, has been identified in different types of cancers including thyroid, lung, breast, liver, colon, kidney, ovary and gastric carcinoma.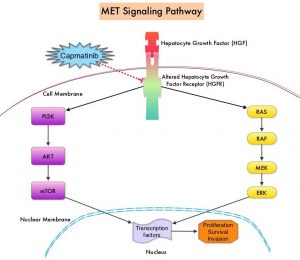
Two key MET alterations include MET exon 14 skipping mutations and MET amplification. MET exon 14 skipping mutations occur in approximately 5% of NSCLC patients with enrichment in sarcomatoid lung cancers (22%). MET exon 14 skipping mutation is a recognized oncogenic driver and is a molecular genetic abnormality indicating the presence of a splice site mutation that results in a loss of transcription of exon 14 of the MET gene. Most exon 14 mutations occur in never-smokers and is seen in both squamous and adenocarcinoma histology. Patients whose cancers have MET exon 14 skipping generally have very high response rates to MET inhibitors and molecular testing for MET exon 14 skipping should therefore be performed on all lung cancers, because this is a targetable alteration. MET amplification has been more commonly seen in smokers, and responses in patients with MET-amplified tumors might be more variable and dependent on level of amplification, with higher responses noted in tumors with more than 5-6 fold amplification. Tumors with MET exon 14 skipping mutations usually do not harbor activating mutations in EGFR, KRAS, or BRAF or concurrent ALK, ROS1 or RET translocations. However, it appears that cMET exon 14 skipping is not mutually exclusive with cMET amplification.
TABRECTA® (Capmatinib) is a highly potent and selective, reversible inhibitor of MET tyrosine kinase. The present FDA approval was based on the primary findings from the Phase II GEOMETRY mono-1 trial, which is a non-randomized, open-label, multi-cohort, Phase II study, conducted to evaluate the efficacy and safety of single-agent TABRECTA® in adult patients with EGFR wild-type, ALK-negative, metastatic NSCLC, whose tumors have a mutation that leads to MET exon 14 skipping (METex14), as detected by an RNA-based RT-PCR. This study enrolled 97 patients with metastatic NSCLC and confirmed MET exon 14 skipping mutations, 69 of whom were previously treated and, 28 of whom, were treatment naive. The patients received TABRECTA® at 400 mg orally twice daily until disease progression or unacceptable toxicity. The median patient age was 71 years and all NSCLC histologies including sarcomatoid/carcinosarcoma were included. Majority of the patients (75%) were white and 24% were Asian. Previous treatments included immunotherapy (28%) and chemotherapy (94%), and 23% of patients received 2 prior lines of therapy. The main efficacy outcome was Overall Response Rate (ORR) and additional efficacy outcomes included Duration of Response, Time to Response, Disease Control Rate, Progression Free Survival (PFS) and Safety. Thirteen patients (N=13) in this study had brain metastases at baseline.
Among the treatment-naïve patients group, the ORR was 68% with a median Duration of Response of 12.6 months and the percentage of patients with responses for 12 months or longer was 47%. The Disease Control Rate (Complete Response plus Partial Response plus Stable Disease) was 96.4%.
Among the previously treated patients, the ORR was 41%, with a median Duration of Response of 9.7 months and the percentage of patients with responses for 12 months or longer was 32%. The Disease Control Rate was 78.3%. Among those with brain metastases at baseline, 54% had an intracranial response with TABRECTA® with 31% showing complete resolution, 23% showing partial resolution, and the intracranial Disease Control Rate was 92%. The most common adverse events (occurring in at least 20% of patients) were peripheral edema, nausea, fatigue, vomiting, dyspnea, and decreased appetite. TABRECTA® can also cause Interstitial Lung Disease, hepatotoxicity and photosensitivity.
It was concluded that TABRECTA® is a new treatment option for patients with MET exon 14 skipping- mutated advanced NSCLC, regardless of the line of therapy, with deep and durable responses, manageable toxicity profile, and is the first and only FDA approved treatment for this patient group.
Capmatinib (INC280) in METex14-mutated advanced non-small cell lung cancer (NSCLC): Efficacy data from the phase II GEOMETRY mono-1 study. Wolf J, Seto T, Han J, et al. J Clin Oncol. 2019;37(suppl; abstr 9004).
TABRECTA® (Capmatinib)
The FDA on May 6,2020 granted accelerated approval to TABRECTA® for adult patients with metastatic Non-Small Cell Lung Cancer (NSCLC) whose tumors have a mutation that leads to Mesenchymal-Epithelial Transition (MET) exon 14 skipping, as detected by an FDA-approved test. TABRECTA® is a product of Novartis.
NELSON Trial Confirms that Low-Dose CT Screening Reduces Lung Cancer Mortality
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2020, about 228, 820 new cases of lung cancer will be diagnosed and 135,720 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of NSCLC, 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large Cell Carcinomas. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, Adenocarcinoma now is the most frequent histologic subtype of lung cancer.
In the National Lung Screening Trial (NLST) with Low Dose CT (LDCT) screening for lung cancer, there was a 20% reduction in mortality. Following the publication of the results of NLST and NCCN issued guideline in 2011, the United States Preventive Services Task Force (USPSTF) recommended Lung Cancer screening with Low Dose CT scan in high risk patients. CMS in 2015 determined that there was sufficient evidence to reimburse for this preventive service.
Despite the evidence and recommendation along with supportive public policies, screening with LDCT has not been adequately implemented in the US healthcare system. Low Health Care Provider knowledge of the Lung Cancer Screening (LCS) guidelines represents a potential barrier to implementation. Additionally, despite the unequivocal findings from the NLST, several countries have not adopted this guideline due to early publication of inconclusive results from a number of smaller trials in Europe. Further, there are limited data from randomized trials regarding whether nodule volume-based, low-dose CT screening can reduce lung cancer mortality among male former and current smokers.
The Dutch-Belgian lung cancer screening trial, NELSON (Nederlands–Leuvens Longkanker Screenings Onderzoek ) is a population-based, randomized, controlled trial initiated in 2000. The goal of the study was to show a 25% or more reduction in lung cancer mortality, in high-risk male participants at 10 years of follow-up, utilizing volume-based, low-dose CT lung cancer screening. The authors in this publication reported the incidence of lung cancer, associated mortality, and the performance of the four rounds of low-dose CT screening for lung cancer in the this trial, among male participants (main analysis) and female participants (subgroup analyses).
In this study, a total of 13,195 men aged 50-74 years were randomly assigned to undergo CT screening at baseline, year 1, year 3, and year 5.5 (N = 6,583) or no screening (N=6,612). At the time of initiation of this trial, only a small number of women were eligible, as smoking was much less prevalent and intense among women, than among men. Because of the relevance and importance of the inclusion of women in this study, high-risk women were later allowed to participate (N=2594). Participants were current or former smokers who had quit 10 or fewer years ago, who had smoked more than 15 cigarettes a day for more than 25 years, or more than 10 cigarettes a day for more than 30 years. About 45% of the male participants were former smokers.
At 10 years of follow up, lung cancer mortality among men was 24% lower in the screening group than in the control group (Rate Ratio=0.76; P=0.01), and 33% lower among women (Rate Ratio=0.67). The CT screening compliance among men was 90% and approximately 9.2% of the screened participants underwent at least one additional CT scan due to an indeterminate screening test. Screening-detected lung cancers were substantially more often diagnosed in Stage IA or IB (58.6%) and were Adenocarcinomas (52.0% in the screening group and 43.8% in the control group).
It was concluded that in this trial involving high-risk individuals, lung cancer mortality was significantly lower among those who underwent CT screening with determination of nodule volume, than among those who underwent no screening. Adherence to CT screening was very high, with low rates of follow up procedures for results suggestive of lung cancer. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. de Koning H.J., van der Aalst C.M., de Jong P.A., et al. N Engl J Med 2020;382:503-513
Lung Immune Prognostic Index (LIPI) is an Important Prognostic Biomarker for Patients with Advanced Non Small Cell Lung Cancer
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2020, about 228, 820 new cases of lung cancer will be diagnosed and 135,720 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of NSCLC, 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large Cell Carcinomas. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, Adenocarcinoma now is the most frequent histologic subtype of lung cancer.
Immunotherapy with PD-1/PD-L1 (Programmed Death-1/Programmed Death-Ligand 1) inhibitors, also called Immune Checkpoint Inhibitors (ICIs), has changed the treatment paradigm for patients with advanced NSCLC. In previously treated patients with NSCLC, the Overall Response Rates (ORR) with single agent Immune Checkpoint Inhibitors (ICIs) range from 14-20%, with median Overall Survival (OS) of 10 to 12 months. In those with PD-L1 expression of 50% or more by ImmunoHistoChemical (IHC) analysis, the ORR can reach up to 30% with a median OS of 20 months. However, in patients with negative or weak PD-L1 expression (1%-49% positive tumor cells), who account for approximately two thirds of the NSCLC population, the response rates range from 8-19% with a median OS slightly below 10 months. Even among those with tumors expressing PD-L1 expression of 50% or more, not all patients benefit from Immunotherapy with ICIs. Therefore identifying biomarkers for patients likely to respond to ICI therapy, and predicting resistance is important and relevant in selecting the appropriate patients for treatment with ICIs.
There is growing evidence on the role of inflammation in cancer biology and systemic inflammatory response may have prognostic significance in different cancer types. Inflammatory process in various cancers imparts immunoresistance to ICIs, by activating oncogenic signaling pathways, there by promoting cancer growth and dissemination, with resulting poor outcomes. Derived Neutrophil-to-Lymphocyte ratio (dNLR) and serum Lactate DeHydrogenase (LDH) level have been investigated as potential inflammatory biomarkers in patients with cancer. The dNLR is calculated using a formula dNLR= Absolute Neutrophil Count/(White Blood Count minus Absolute Neutrophil Count). These ratios are simple and easy to calculate from routine blood tests. Both these biomarkers have been correlated with Immune Checkpoint Inhibitor outcomes, in patients with melanoma. In two large studies involving patients with advanced melanoma treated with Ipilimumab and Pembrolizumab, dNLR of 3 or more and LDH of at least 2.5 times Upper Limit of Normal (ULN), reflected a pro-inflammatory status and resulted in poor outcomes.
Based on this important finding in malignant melanoma, Mezquita L and colleagues (JAMA Oncol. 2018;4:351-357) conducted a multicenter, retrospective study involving 466 patients treated with ICIs, to determine whether combining the two factors – pretreatment dNLR and LDH (Lung Immune Prognostic Index-LIPI), was associated with resistance to ICIs in patients with advanced NSCLC. In this study, LIPI was developed on the basis of dNLR (derived Neutrophil-to-Lymphocyte Ratio) of greater than 3 and LDH greater than Upper Limit of Normal (ULN). LIPI was used to stratify patients with NSCLC into 3 groups (Good= 0 factors; Intermediate= 1 of 2 factors, Poor= 2 factors). The authors based on this study concluded that pretreatment LIPI, combining derived Neutrophil-to-Lymphocyte ratio (dNLR) greater than 3 and serum LDH level greater than Upper Limit of Normal, correlated with worse outcomes for Immune Checkpoint Inhibitors (ICIs).
To determine whether LIPI score provides prognostic information for patients with metastatic NSCLC, the authors in this publication performed an exploratory retrospective analysis of the LIPI on pooled clinical trial data from 11 randomized multinational studies (5 ICI trials and 6 targeted therapy trials), and in the final analysis included 3987 patients treated with ICIs, targeted therapy, or cytotoxic chemotherapy, between January 1, 2013, and December 31, 2017. In the 5 ICI trials (N = 2440), 1368 patients received ICIs and 1072 received cytotoxic chemotherapy. In the 6 targeted therapy trials (N = 1547), 53% of EGFR mutant and 47.1% of ALK positive patients received targeted therapy 32.0% of EGFR mutant and 68% of ALK positive patients received cytotoxic chemotherapy. Baseline demographics and disease characteristics were relatively balanced between groups. Lung Immune Prognostic Index (LIPI) scores were calculated based on the dNLR and the LDH level, as mentioned elsewhere in this document.
For patients receiving ICIs, a good LIPI score was associated with longer Overall Survival (OS) compared with a poor LIPI score, with an estimated median survival of 15.6 versus 4.5 months (HR=0.34). A similar prognostic association was observed for patients who received cytotoxic chemotherapy, with patients having a good LIPI score having a longer survival than patients with a poor score, with an estimated median survival of 10.4 versus 5.3 months (HR=0.49). Similar associations were also noted between good LIPI scores and longer Progression Free Survival (PFS). As expected, PD-L1 expression of 1% or more, as well as higher albumin levels was independently associated with improved outcomes.
Among patients with tumors harboring either ALK alterations or EGFR-activating mutations who received targeted therapy, those with a good LIPI score had an estimated median survival of 46.5 months compared with 16.6 months for those with a poor score (HR=0.28). A similar prognostic association was observed in this patient group receiving cytotoxic chemotherapy, with patients having a good LIPI score experiencing a longer survival than patients with a poor score (estimated median survival of 33.4 months versus 17.1 months (HR=0.41). Further, similar associations between LIPI score and PFS were observed. For patients enrolled in these studies, regardless of receiving targeted therapy or cytotoxic chemotherapy, multivariable analysis consistently showed that LIPI score was independently associated with OS and PFS.
It was concluded from this analysis that pretreatment LIPI risk score may be an important prognostic biomarker, irrespective of pharmacologic class of treatment, for patients with metastatic NSCLC. Prognostic Value of the Lung Immune Prognostic Index for Patients Treated for Metastatic Non–Small Cell Lung Cancer. Kazandjian D, Gong Y, Keegan P, et al. JAMA Oncol. 2019;5:1481-1485.
TECENTRIQ® (Atezolizumab)
The FDA on December 3, 2019 approved TECENTRIQ® in combination with Paclitaxel protein-bound and Carboplatin for the first-line treatment of adult patients with metastatic non-squamous Non-Small Cell Lung Cancer (NSCLC) with no EGFR or ALK genomic tumor aberrations. TECENTRIQ® is a product of Genentech Inc.
