Written by Dr. Solly S. Chedid
Sponsored and developed by Boehringer Ingelheim Pharmaceuticals.
Immunotherapies have changed the way we initiate treatment for many patients with advanced squamous non-small cell lung cancer (NSCLC).1 As immunotherapy has become a standard first-line treatment, non-immunotherapy options are important to consider for second-line treatment. Currently, there is no clear standard of care for second-line therapy in patients with advanced squamous NSCLC who progress after immuno-chemotherapy. Therefore, an unmet need remains for studies designed to understand the effectiveness and safety of second-line treatments in these patients. Here we will review newly published real-world evidence on second-line treatments of patients with squamous NSCLC with afatinib (GILOTRIF®) following immuno-chemotherapy.
GILOTRIF is the only oral, chemotherapy-free option for treating patients with squamous NSCLC that has progressed after platinum-based chemotherapy.2 The efficacy and safety of GILOTRIF were demonstrated in the pivotal LUX-Lung 8 trial. In LUX-Lung 8, treatment with GILOTRIF led to statistically significant improvements in progression-free survival (median 2.4 vs 1.9 months) and overall survival (median 7.9 vs 6.8 months) compared with erlotinib. In LUX-Lung 8, the most common adverse reactions reported in the GILOTRIF treated patients (≥20% all grades) were diarrhea (75%), rash/acneiform dermatitis (70%), stomatitis (30%), decreased appetite (25%), and nausea (21%).
The Real-world Effectiveness of 2L Treatment of Squamous mNSCLC Study is the first to evaluate the real-world use of GILOTRIF following first-line immuno-chemotherapy in patients with squamous NSCLC.1 It is a retrospective, non-interventional, multisite cohort study using electronic medical records of patients with advanced or metastatic squamous NSCLC treated with pembrolizumab and platinum-doublet chemotherapy in the first line. Patients were treated with either GILOTRIF or physician’s choice chemotherapy in the second line. Study endpoints included patient demographics and clinical characteristics, time on second-line treatment, and incidence of severe (Grade ≥3) immune-related adverse events (irAEs). This study analysis was not powered to compare characteristics or outcomes between the cohorts. In addition, the results of this study are not intended for direct comparison with clinical trials. The main limitations of this study are its retrospective nature, potential for selection bias, and lack of a comparator arm.
A total of 200 patients were included in this study; 99 received GILOTRIF, and 101 received chemotherapy in the second line.1 More patients in the GILOTRIF cohort had mixed histology, were epidermal growth factor receptor (EGFR) mutation−positive, and were never smokers than those in the chemotherapy cohort. There were geographic differences between the cohorts; more patients from the Northeast received GILOTRIF, and more patients from the South received chemotherapy. In the GILOTRIF cohort, 45% of patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) ranging from 0 to 1, while 55% had an ECOG PS of 2 or higher. In the chemotherapy arm, 50% of patients had ECOG PS 0 to 1, and 50% had ECOG PS of 2 or higher. Other characteristics, such as median age and stage at diagnosis, were similar in both cohorts.
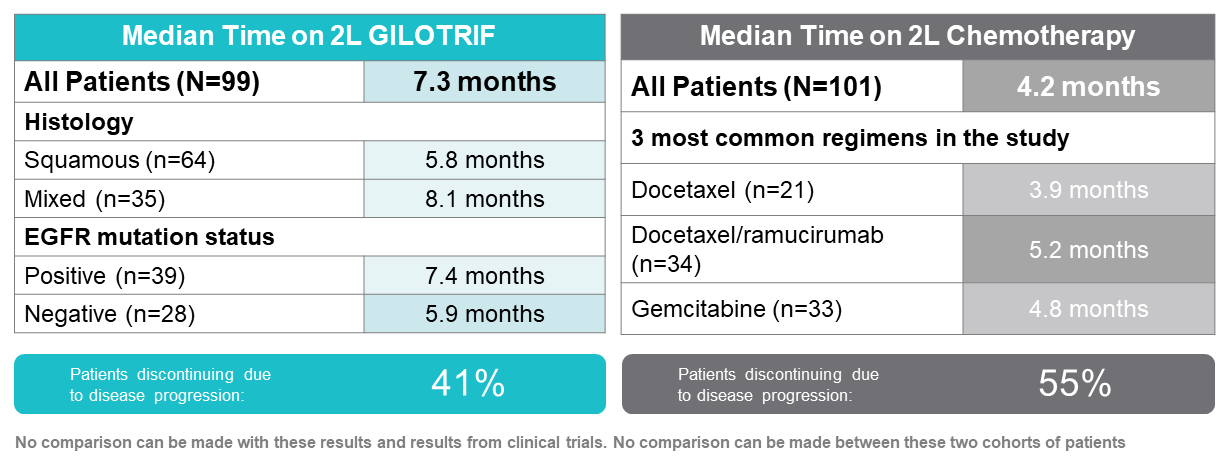
The median time on treatment for the GILOTRIF cohort was 7.3 months. In patients with mixed histology, the median time on treatment was 8.1 months, and for patients with squamous histology it was 5.8 months.1 EGFR mutation−positive and EGFR mutation−negative patients remained on GILOTRIF for a median of 7.4 and 5.9 months, respectively. The median time on treatment from initiation of second-line chemotherapy was 4.2 months.
Time on Treatment in the Real-World Effectiveness Study1
The most common adverse drug reactions with GILOTRIF were diarrhea (26%), rash (6%), stomatitis, fatigue, and nausea (5% each).1 Six out of 99 patients experienced a Grade 3/4 irAE during second-line GILOTRIF therapy; each of these patients also experienced a Grade 3 irAE during first-line treatment. The 6 patients in the GILOTRIF cohort who experienced Grade 3/4 irAEs were treated with steroids, and none were hospitalized. Given the real-world nature of the study, adverse event data may be underreported or underdocumented; in addition, censoring may also bias results.
Such real-world evidence (RWE) studies have limitations, including their retrospective nature and potential for selection bias.1 However, in addition to clinical data collected in registrational clinical trials, data from RWE studies such as this can add important information to help evaluate the clinical utility of a drug in the real-world setting.3 RWE studies can be derived from rich data sources, such as electronic health records, registries, and claims databases, which reflect real-world use, outcomes, and the patient diversity seen in clinical practice.
Despite several limitations highlighted in this paper, the study adds to the body of evidence supporting the effectiveness and safety of GILOTRIF when given as a second-line treatment following immuno-chemotherapy in routine clinical practice.1
INDICATION AND USAGE
GILOTRIF is indicated for the treatment of patients with metastatic squamous NSCLC progressing after platinum-based chemotherapy.
IMPORTANT SAFETY INFORMATION FOR GILOTRIF® (AFATINIB) TABLETS
WARNINGS AND PRECAUTIONS
Diarrhea
• GILOTRIF can cause diarrhea which may be severe and can result in dehydration with or without renal impairment. In clinical studies, some of these cases were fatal.
• For patients who develop Grade 2 diarrhea lasting more than 48 hours or Grade 3 or greater diarrhea, withhold GILOTRIF until diarrhea resolves to Grade 1 or less, and then resume at a reduced dose.
• Provide patients with an anti-diarrheal agent (e.g., loperamide) for self-administration at the onset of diarrhea and instruct patients to continue anti-diarrheal until loose stools cease for 12 hours.
Bullous and Exfoliative Skin Disorders
• GILOTRIF can result in cutaneous reactions consisting of rash, erythema, and acneiform rash. In addition, palmar-plantar erythrodysesthesia syndrome was observed in clinical trials in patients taking GILOTRIF.
• Discontinue GILOTRIF in patients who develop life-threatening bullous, blistering, or exfoliating skin lesions. For patients who develop Grade 2 cutaneous adverse reactions lasting more than 7 days, intolerable Grade 2, or Grade 3 cutaneous reactions, withhold GILOTRIF. When the adverse reaction resolves to Grade 1 or less, resume GILOTRIF with appropriate dose reduction.
• Postmarketing cases of toxic epidermal necrolysis (TEN) and Stevens Johnson syndrome (SJS) have been reported in patients receiving GILOTRIF. Discontinue GILOTRIF if TEN or SJS is suspected.
Interstitial Lung Disease
• Interstitial Lung Disease (ILD) or ILD-like adverse reactions (e.g., lung infiltration, pneumonitis, acute respiratory distress syndrome, or alveolitis allergic) occurred in patients receiving GILOTRIF in clinical trials. In some cases, ILD was fatal. The incidence of ILD appeared to be higher in Asian patients as compared to white patients.
• Withhold GILOTRIF during evaluation of patients with suspected ILD, and discontinue GILOTRIF in patients with confirmed ILD.
Hepatic Toxicity
• Hepatic toxicity as evidenced by liver function tests abnormalities has been observed in patients taking GILOTRIF. In 4257 patients who received GILOTRIF across clinical trials, 9.7% had liver test abnormalities, of which 0.2% were fatal.
• Obtain periodic liver testing in patients during treatment with GILOTRIF. Withhold GILOTRIF in patients who develop worsening of liver function. Discontinue treatment in patients who develop severe hepatic impairment while taking GILOTRIF.
Gastrointestinal Perforation
• Gastrointestinal (GI) perforation, including fatal cases, has occurred with GILOTRIF. GI perforation has been reported in 0.2% of patients treated with GILOTRIF among 3213 patients across 17 randomized controlled clinical trials.
• Patients receiving concomitant corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), or anti-angiogenic agents, or patients with increasing age or who have an underlying history of GI ulceration, underlying diverticular disease, or bowel metastases may be at an increased risk of perforation.
• Permanently discontinue GILOTRIF in patients who develop GI perforation.
Keratitis
• Keratitis has been reported in patients taking GILOTRIF.
• Withhold GILOTRIF during evaluation of patients with suspected keratitis. If diagnosis of ulcerative keratitis is confirmed, interrupt or discontinue GILOTRIF. If keratitis is diagnosed, the benefits and risks of continuing treatment should be carefully considered. GILOTRIF should be used with caution in patients with a history of keratitis, ulcerative keratitis, or severe dry eye. Contact lens use is also a risk factor for keratitis and ulceration.
Embryo-Fetal Toxicity
• GILOTRIF can cause fetal harm when administered to a pregnant woman. Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
• Advise females of reproductive potential to use effective contraception during treatment, and for at least 2 weeks after the last dose of GILOTRIF. Advise female patients to contact their healthcare provider with a known or suspected pregnancy.
ADVERSE REACTIONS
Adverse Reactions observed in clinical trials were as follows:
Previously Treated, Metastatic Squamous NSCLC
• In GILOTRIF-treated patients (n=392) the most common adverse reactions (≥20% all grades & vs erlotinib-treated patients (n=395)) were diarrhea (75% vs 41%), rash/acneiform dermatitis (70% vs 70%), stomatitis (30% vs 11%), decreased appetite (25% vs 26%), and nausea (21% vs 16%).
• Serious adverse reactions were reported in 44% of patients treated with GILOTRIF. The most frequent serious adverse reactions reported in patients treated with GILOTRIF were pneumonia (6.6%), diarrhea (4.6%), and dehydration and dyspnea (3.1% each). Fatal adverse reactions in GILOTRIF-treated patients included ILD (0.5%), pneumonia (0.3%), respiratory failure (0.3%), acute renal failure (0.3%), and general physical health deterioration (0.3%).
DRUG INTERACTIONS
Effect of P-glycoprotein (P-gp) Inhibitors and Inducers
• Concomitant use of P-gp inhibitors (including but not limited to ritonavir, cyclosporine A, ketoconazole, itraconazole, erythromycin, verapamil, quinidine, tacrolimus, nelfinavir, saquinavir, and amiodarone) with GILOTRIF can increase exposure to afatinib.
• Concomitant use of P-gp inducers (including but not limited to rifampicin, carbamazepine, phenytoin, phenobarbital, and St. John’s wort) with GILOTRIF can decrease exposure to afatinib.
USE IN SPECIFIC POPULATIONS
Lactation
• Because of the potential for serious adverse reactions in breastfed infants from GILOTRIF, advise women not to breastfeed during treatment with GILOTRIF and for 2 weeks after the final dose.
Females and Males of Reproductive Potential
• GILOTRIF may reduce fertility in females and males of reproductive potential. It is not known if the effects on fertility are reversible.
Renal Impairment
• Patients with severe renal impairment (estimated glomerular filtration rate [eGFR] 15 to 29 mL/min/1.73 m2) have a higher exposure to afatinib than patients with normal renal function. Administer GILOTRIF at a starting dose of 30 mg once daily in patients with severe renal impairment. GILOTRIF has not been studied in patients with eGFR <15 mL/min/1.73 m2 or who are on dialysis.
Hepatic Impairment
• GILOTRIF has not been studied in patients with severe (Child Pugh C) hepatic impairment. Closely monitor patients with severe hepatic impairment and adjust GILOTRIF dose if not tolerated.
GF PROF ISI 10.21.19
References
1. Kim ES, Kish JK, Cseh A, et al. Second-line afatinib or chemotherapy following immunochemotherapy for the treatment of metastatic, squamous cell carcinoma of the lung: real-world effectiveness and safety from a multisite retrospective chart review in the USA. Clin Lung Cancer. 2021;S1525-7304(21)00029-2.
2. GILOTRIF. Prescribing information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2019.
3. Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence — what is it and what can it tell us? N Engl J Med. 2016:8;375(23):2293-2297.
Please review the Full Prescribing Information and Patient Information.