By Dr. David Waterhouse | Sponsored by Bristol Myers Squibb
Dr. Waterhouse is a paid consultant for Bristol Myers Squibb and was compensated for his role in drafting this article.
The American Cancer Society estimates that there will be nearly 229,000 new cases of lung cancer in the United States (US) alone in 2020 and nearly 136,000 lung cancer deaths.1 Historically, most patients present with metastatic disease and their long-term outlook is grim.2 However, significant progress has been made in recent years. In August 2020, Howlader et al reported that the population-level mortality from non-small cell lung cancer (NSCLC) in the US fell sharply from 2013 to 2016.3
Based on the results from Checkmate 227 Part 1a, OPDIVO, in combination with YERVOY, is indicated for the first-line treatment of adult patients with metastatic NSCLC whose tumors express PD-L1 (≥1%) as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations.4-6 In addition, based on the results from Checkmate 9LA, OPDIVO, in combination with YERVOY and 2 cycles of platinum-doublet chemotherapy (chemo), is indicated for the first-line treatment of adult patients with metastatic or recurrent NSCLC, with no EGFR or ALK genomic tumor aberrations.4,6,7
OPDIVO and YERVOY are associated with the following Warnings and Precautions: severe and fatal immune-mediated reactions including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis with renal dysfunction, dermatologic adverse reactions, other immune-mediated adverse reactions; infusion-related reactions; complications of allogeneic hematopoietic stem cell transplantation (HSCT); embryo-fetal toxicity; and increased mortality in patients with multiple myeloma when OPDIVO is added to a thalidomide analogue and dexamethasone, which is not recommended outside of controlled clinical trials.4 Please see additional Important Safety Information for OPDIVO and YERVOY at the end of the article and US Full Prescribing Information for OPDIVO and YERVOY at https://packageinserts.bms.com/pi/pi_opdivo.pdf and https://packageinserts.bms.com/pi/pi_yervoy.pdf.
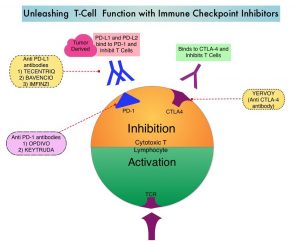
OPDIVO® (nivolumab) is a monoclonal antibody targeting programmed death receptor-1 (PD-1) that has been approved for the treatment of lung cancer.4 YERVOY® (ipilimumab) is another monoclonal antibody that works to activate the immune system by targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4).6,8
Figure 1: OPDIVO and YERVOY mechanisms of action4,6,8-14
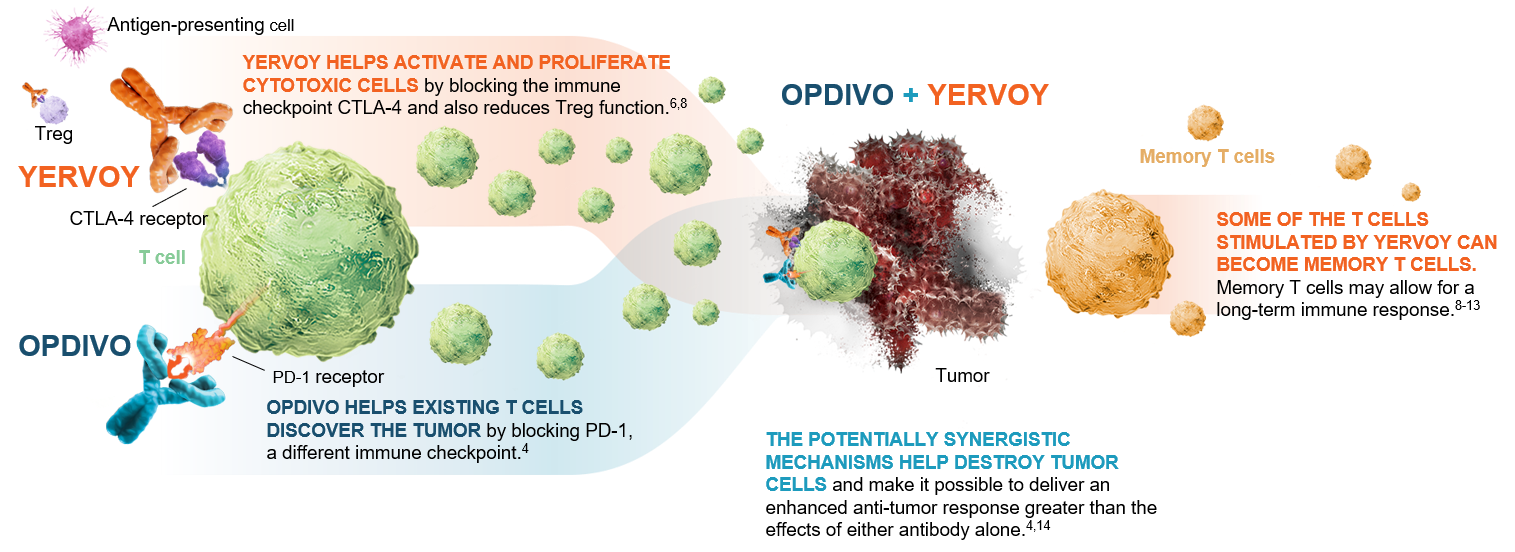 This graphic is for demonstration purposes only.
This graphic is for demonstration purposes only.
The illustrated mechanisms may vary for each patient and may not directly correlate with clinical significance.
The phase 3 Checkmate 227 and Checkmate 9LA trials investigated OPDIVO plus YERVOY-based combinations for first-line treatment of certain NSCLC patients.4 Part 1a of Checkmate 227 investigated the effects of OPDIVO + YERVOY compared with standard chemo* among patients whose tumors expressed ≥1% programmed death ligand 1 (PD-L1)4 (Figure 2).
Figure 2: Checkmate 227 Part 1a study design15
 *In Checkmate 227, patients in the comparator arm received up to 4 cycles of platinum-doublet chemo q3w; NSQ: pemetrexed + carboplatin or cisplatin, with optional pemetrexed maintenance following chemo; SQ: gemcitabine + carboplatin or cisplatin.4,16,17
*In Checkmate 227, patients in the comparator arm received up to 4 cycles of platinum-doublet chemo q3w; NSQ: pemetrexed + carboplatin or cisplatin, with optional pemetrexed maintenance following chemo; SQ: gemcitabine + carboplatin or cisplatin.4,16,17
ALK=anaplastic lymphoma kinase; DOR=duration of response; ECOG PS=Eastern Cooperative Oncology Group Performance Status; EGFR=epidermal growth factor receptor; NSQ=non-squamous; q2w=every 2 weeks; q6w=every 6 weeks; SQ=squamous.
OPDIVO + YERVOY showed a superior survival benefit compared with chemo*, with the primary analysis at a minimum follow-up of 29.3 months revealing a median overall survival (OS) of 17.1 months vs 14.9 months with chemo*, and a hazard ratio (HR) of 0.79, 95% confidence interval (CI): 0.67–0.94, P=0.0066 (Figure 3).4,16 The median progression-free survival (PFS) was 5.1 months (95% CI: 4.1–6.3) with OPDIVO + YERVOY and 5.6 months (95% CI: 4.6–5.8) with chemo* alone (HR=0.82; 95% CI: 0.69–0.97).4
The most frequent (≥2%) serious adverse reactions were pneumonia, diarrhea/colitis, pneumonitis, hepatitis, pulmonary embolism, adrenal insufficiency, and hypophysitis. Fatal adverse reactions occurred in 1.7% of patients; these included events of pneumonitis (4 patients), myocarditis, acute kidney injury, shock, hyperglycemia, multi-system organ failure, and renal failure.4 The most common (≥20%) adverse reactions were fatigue (44%), rash (34%), decreased appetite (31%), musculoskeletal pain (27%), diarrhea/colitis (26%), dyspnea (26%), cough (23%), hepatitis (21%), nausea (21%), and pruritus (21%).4 Please continue reading for more Important Safety Information for OPDIVO and YERVOY throughout.
Figure 3: Checkmate 227 OS for PD L1 ≥1% (extended 3-year follow-up analysis)4,15
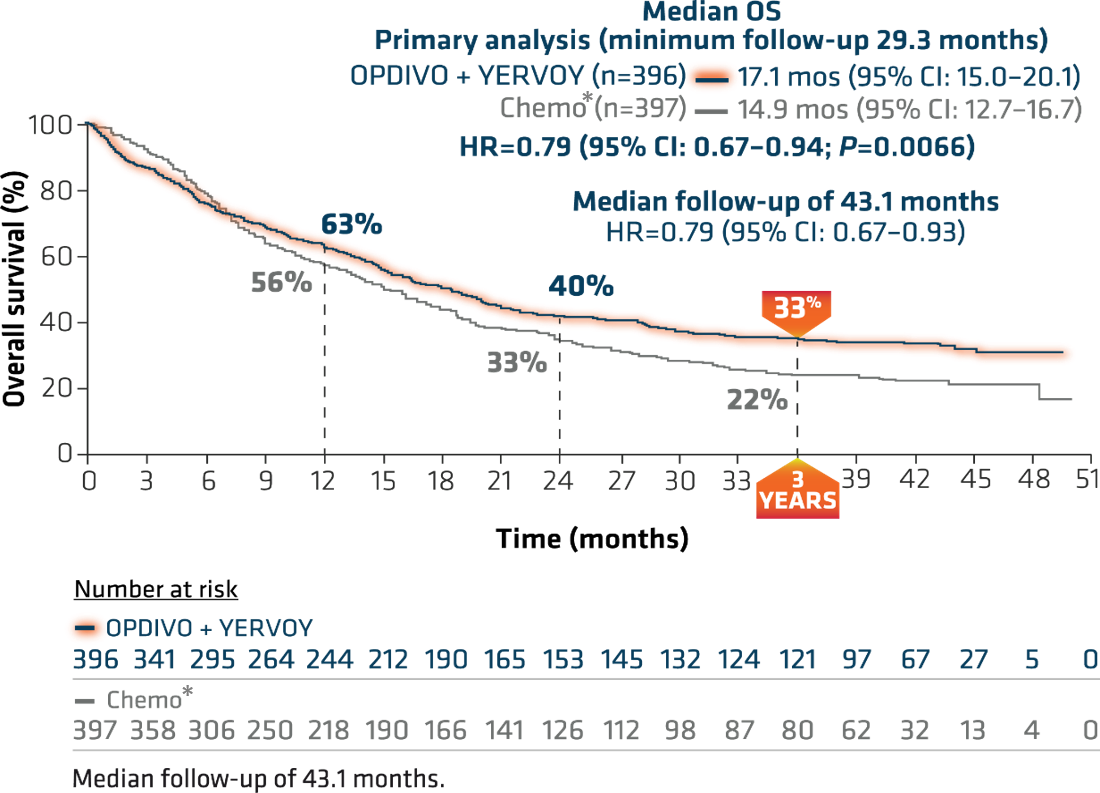
*In Checkmate 227, patients in the comparator arm received up to 4 cycles of platinum-doublet chemo q3w; NSQ: pemetrexed + carboplatin or cisplatin, with optional pemetrexed maintenance following chemo; SQ: gemcitabine + carboplatin or cisplatin.4,16,17
At the American Society for Clinical Oncology (ASCO) 2020 Annual Meeting, 3-year follow-up results from this trial were reported. With a median follow-up of more than 3 years (43.1 months), this study represents the longest median follow-up of any dual immuno-oncology (I-O)-based combination in a phase 3 clinical trial in NSCLC.15 This extended follow-up analysis showed 3-year OS rates of 33% for OPDIVO + YERVOY and 22% for chemo* (Figure 3).15
At minimum follow-up of 28.3 months, the objective response rate was 36% (95% CI: 31–41), CR=5.8%, PR=30.1% with OPDIVO + YERVOY and 30% (95% CI: 26–35), CR=1.8%, PR=28.2% with chemo*.4,16,17 The median duration of response from the extended 3-year follow-up analysis was 23.2 months (95% CI: 15.2–32.2) in patients who responded to OPDIVO + YERVOY and 6.7 months (95% CI: 5.6–7.6) with chemo* (Figure 4).15
Figure 4: Checkmate 227 DOR among responders with PD L1 ≥1% (extended 3-year follow-up analysis)15
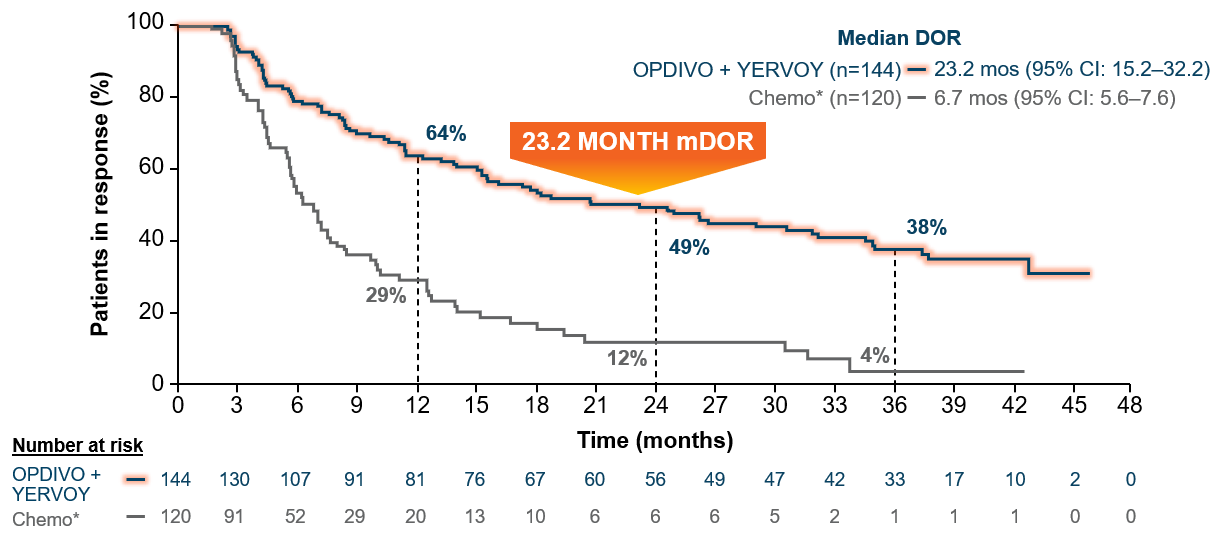
Median follow-up of 43.1 months.15
*In Checkmate 227, patients in the comparator arm received up to 4 cycles of platinum-doublet chemo q3w; NSQ: pemetrexed + carboplatin or cisplatin, with optional pemetrexed maintenance following chemo; SQ: gemcitabine + carboplatin or cisplatin.4,16,17
The 3-year data from Checkmate 227 Part 1a show the long-term durable survival of a dual immunotherapy approach.15 The FDA approved OPDIVO + YERVOY on May 15, 2020, for first-line treatment of adult patients with metastatic NSCLC whose tumors express PD-L1(≥1%) as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations. With this approval, these patients with NSCLC can now be offered the option of dual I-O therapy.4,5
Also reported at ASCO 2020 were the results of Checkmate 9LA.18 Patients were randomized to receive either the combination of OPDIVO + YERVOY and 2 cycles of platinum-doublet chemo† or platinum-doublet chemo† for 4 cycles.4 This trial evaluated patients regardless of PD-L1 expression and histology (Figure 5).4
Figure 5: Checkmate 9LA study design18
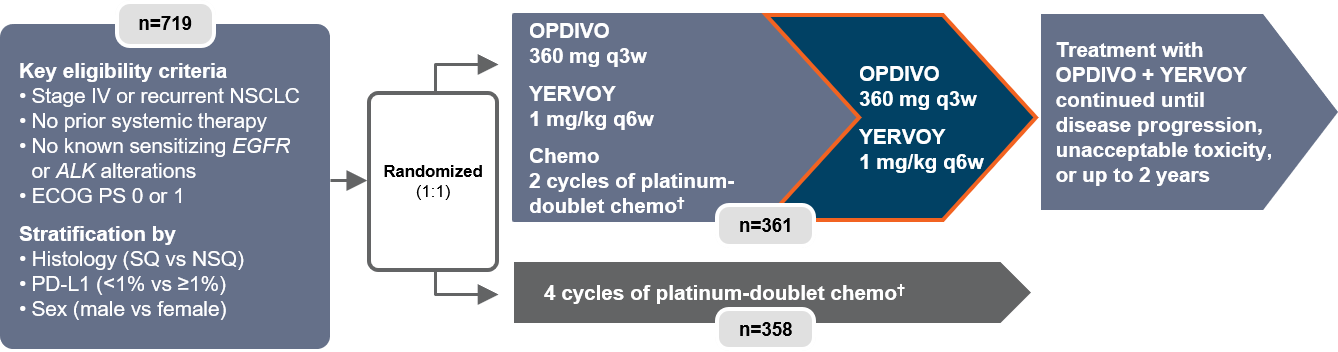
†In Checkmate 9LA, patients received 2 cycles of platinum-doublet chemo q3w in the experimental arm, and up to 4 cycles in the comparator arm; NSQ: pemetrexed + carboplatin or cisplatin (optional pemetrexed maintenance therapy in comparator arm only); SQ: paclitaxel + carboplatin.4
q3w=every three weeks.
The trial showed a superior benefit in OS for patients treated with OPDIVO + YERVOY with limited chemo† compared to those who received chemo† alone.18 At the pre-specified interim analysis at 8.1 months, the median OS was 14.1 months vs 10.7 months (HR=0.69, 96.71% CI: 0.55-0.87, P=0.0006).4 Median PFS per blinded independent central review (BICR) at minimum follow-up of 6.5 months was 6.8 months among patients who received OPDIVO + YERVOY with chemo†, and 5.0 months among patients receiving chemo† (HR=0.70, 97.48% CI: 0.57-0.86).4 Confirmed ORR per BICR at minimum follow-up of 6.5 months was 38% (95% CI: 33-43) and 25% (95% CI: 21-30) respectively.4,18
A follow-up analysis performed at 12.7 months showed median OS of 15.6 months with OPDIVO + YERVOY with chemo† and 10.9 months with chemo† alone with HR of 0.66 (95% CI: 0.55-0.80) (Figure 6).4,18 OS was consistent across PD-L1 expression levels at minimum follow-up of 8.1 months, with median OS of 14.0 months (95% CI:13.2-NR) and 10.0 months (95% CI: 7.7-13.7) in patients treated with OPDIVO + YERVOY with limited chemo† and chemo† respectively in the PD-L1 <1% sub-population (HR=0.65), and median OS of 14.2 months (95% CI:13.1-NR) and 10.6 months (95% CI: 9.4-12.6) respectively (HR=0.67) in the PD-L1 ≥1% sub-population.19
Figure 6: Checkmate 9LA overall survival (extended follow-up)18
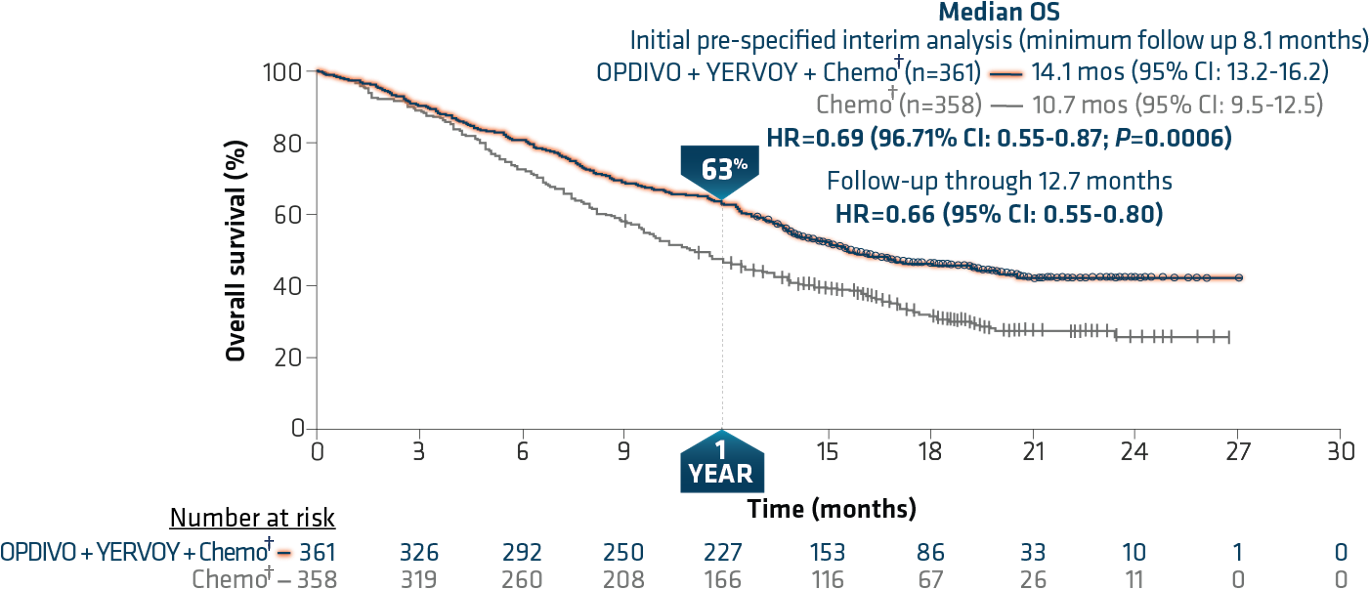
Minimum follow-up of 12.7 months.
†In Checkmate 9LA, patients received 2 cycles of platinum-doublet chemo q3w in the experimental arm, and up to 4 cycles in the comparator arm; NSQ: pemetrexed + carboplatin or cisplatin (optional pemetrexed maintenance therapy in comparator arm only); SQ: paclitaxel + carboplatin.4
In this study, the most frequent (>2%) serious adverse reactions were pneumonia, diarrhea, febrile neutropenia, anemia, acute kidney injury, musculoskeletal pain, dyspnea, pneumonitis, and respiratory failure. Fatal adverse reactions occurred in 7 (2%) patients, and included hepatic toxicity, acute renal failure, sepsis, pneumonitis, diarrhea with hypokalemia, and massive hemoptysis in the setting of thrombocytopenia.4 The most common (>20%) adverse reactions were fatigue (49%), musculoskeletal pain (39%), nausea (32%), diarrhea (31%), rash (30%), decreased appetite (28%), constipation (21%), and pruritus (21%).4 Please continue reading for more Important Safety Information for OPDIVO and YERVOY throughout. The FDA approved OPDIVO, in combination with YERVOY and 2 cycles of platinum-doublet chemo, for the first-line treatment of adult patients with metastatic or recurrent NSCLC with no EGFR or ALK genomic tumor aberrations in May 2020.4,7
With the approval of both Checkmate 227 and Checkmate 9LA regimens as first-line therapies, I am pleased to be able to offer metastatic NSCLC patients with additional options. Checkmate 227 provides appropriate mNSCLC patients with a chemo-free, dual I-O option with long-term, durable survival. Additionally, the Checkmate 9LA regimen with dual I-O plus limited chemo† has shown superior OS, and consistent OS, regardless of PD-L1 expression in recurrent/metastatic NSCLC patients.4,18
*In Checkmate 227, patients in the comparator arm received up to 4 cycles of platinum-doublet chemo q3w; NSQ: pemetrexed + carboplatin or cisplatin, with optional pemetrexed maintenance following chemo; SQ: gemcitabine + carboplatin or cisplatin.4,16,17
†In Checkmate 9LA, patients received 2 cycles of platinum-doublet chemo q3w in the experimental arm, and up to 4 cycles in the comparator arm; NSQ: pemetrexed + carboplatin or cisplatin (optional pemetrexed maintenance therapy in comparator arm only); SQ: paclitaxel + carboplatin.4
IMPORTANT SAFETY INFORMATION
Severe and Fatal Immune-Mediated Adverse Reactions
Immune-mediated adverse reactions listed herein may not include all possible severe and fatal immune-mediated adverse reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. While immune-mediated adverse reactions usually manifest during treatment, they can also occur after discontinuation of OPDIVO or YERVOY. Early identification and management are essential to ensure safe use of OPDIVO and YERVOY. Monitor for signs and symptoms that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine, adrenocorticotropic hormone (ACTH) level, and thyroid function at baseline and periodically during treatment with OPDIVO and before each dose of YERVOY. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue OPDIVO and YERVOY depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). In general, if OPDIVO or YERVOY interruption or discontinuation is required, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy. Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
OPDIVO and YERVOY can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation. In NSCLC patients receiving OPDIVO 3 mg/kg every 2 weeks with YERVOY 1 mg/kg every 6 weeks, immune-mediated pneumonitis occurred in 9% (50/576) of patients, including Grade 4 (0.5%), Grade 3 (3.5%), and Grade 2 (4.0%). Four patients (0.7%) died due to pneumonitis.
Immune-Mediated Colitis
OPDIVO and YERVOY can cause immune-mediated colitis, which may be fatal. A common symptom included in the definition of colitis was diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
Immune-Mediated Hepatitis
OPDIVO and YERVOY can cause immune-mediated hepatitis.
Immune-Mediated Endocrinopathies
OPDIVO and YERVOY can cause primary or secondary adrenal insufficiency, immune-mediated hypophysitis, immune-mediated thyroid disorders, and Type 1 diabetes mellitus, which can present with diabetic ketoacidosis. Withhold OPDIVO and YERVOY depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism; initiate hormone replacement as clinically indicated. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism; initiate hormone replacement or medical management as clinically indicated. Monitor patients for hyperglycemia or other signs and symptoms of diabetes; initiate treatment with insulin as clinically indicated.
Immune-Mediated Nephritis with Renal Dysfunction
OPDIVO and YERVOY can cause immune-mediated nephritis.
Immune-Mediated Dermatologic Adverse Reactions
OPDIVO can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS) has occurred with PD-1/PD-L1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate nonexfoliative rashes.
YERVOY can cause immune-mediated rash or dermatitis, including bullous and exfoliative dermatitis, SJS, TEN, and DRESS. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-bullous/exfoliative rashes.
Withhold or permanently discontinue OPDIVO and YERVOY depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information).
Other Immune-Mediated Adverse Reactions
The following clinically significant immune-mediated adverse reactions occurred at an incidence of <1% (unless otherwise noted) in patients who received OPDIVO monotherapy or OPDIVO in combination with YERVOY or were reported with the use of other PD-1/PD-L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions: cardiac/vascular: myocarditis, pericarditis, vasculitis; nervous system: meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy; ocular: uveitis, iritis, and other ocular inflammatory toxicities can occur; gastrointestinal: pancreatitis to include increases in serum amylase and lipase levels, gastritis, duodenitis; musculoskeletal and connective tissue: myositis/polymyositis, rhabdomyolysis, and associated sequelae including renal failure, arthritis, polymyalgia rheumatica; endocrine: hypoparathyroidism; other (hematologic/immune): hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis (HLH), systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection.
In addition to the immune-mediated adverse reactions listed above, across clinical trials of YERVOY monotherapy or in combination with OPDIVO, the following clinically significant immune-mediated adverse reactions, some with fatal outcome, occurred in <1% of patients unless otherwise specified: nervous system: autoimmune neuropathy (2%), myasthenic syndrome/myasthenia gravis, motor dysfunction; cardiovascular: angiopathy, temporal arteritis; ocular: blepharitis, episcleritis, orbital myositis, scleritis; gastrointestinal: pancreatitis (1.3%); other (hematologic/immune): conjunctivitis, cytopenias (2.5%), eosinophilia (2.1%), erythema multiforme, hypersensitivity vasculitis, neurosensory hypoacusis, psoriasis.
Some ocular IMAR cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada–like syndrome, which has been observed in patients receiving YERVOY, as this may require treatment with systemic corticosteroids to reduce the risk of permanent vision loss.
Infusion-Related Reactions
OPDIVO and YERVOY can cause severe infusion-related reactions. Discontinue OPDIVO and YERVOY in patients with severe (Grade 3) or life-threatening (Grade 4) infusion-related reactions. Interrupt or slow the rate of infusion in patients with mild (Grade 1) or moderate (Grade 2) infusion-related reactions.
Complications of Allogeneic Hematopoietic Stem Cell Transplantation
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with OPDIVO or YERVOY. Transplant-related complications include hyperacute graft-versus-host-disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between OPDIVO or YERVOY and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with OPDIVO and YERVOY prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal studies, OPDIVO and YERVOY can cause fetal harm when administered to a pregnant woman. The effects of YERVOY are likely to be greater during the second and third trimesters of pregnancy. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with OPDIVO and YERVOY and for at least 5 months after the last dose.
Increased Mortality in Patients with Multiple Myeloma when OPDIVO is Added to a Thalidomide Analogue and Dexamethasone
In randomized clinical trials in patients with multiple myeloma, the addition of OPDIVO to a thalidomide analogue plus dexamethasone resulted in increased mortality. Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials.
Lactation
There are no data on the presence of OPDIVO or YERVOY in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment and for 5 months after the last dose.
Serious Adverse Reactions
In Checkmate 227, serious adverse reactions occurred in 58% of patients (n=576). The most frequent (≥2%) serious adverse reactions were pneumonia, diarrhea/colitis, pneumonitis, hepatitis, pulmonary embolism, adrenal insufficiency, and hypophysitis. Fatal adverse reactions occurred in 1.7% of patients; these included events of pneumonitis (4 patients), myocarditis, acute kidney injury, shock, hyperglycemia, multi-system organ failure, and renal failure. In Checkmate 9LA, serious adverse reactions occurred in 57% of patients (n=358). The most frequent (>2%) serious adverse reactions were pneumonia, diarrhea, febrile neutropenia, anemia, acute kidney injury, musculoskeletal pain, dyspnea, pneumonitis, and respiratory failure. Fatal adverse reactions occurred in 7 (2%) patients, and included hepatic toxicity, acute renal failure, sepsis, pneumonitis, diarrhea with hypokalemia, and massive hemoptysis in the setting of thrombocytopenia.
Common Adverse Reactions
In Checkmate 227, the most common (≥20%) adverse reactions were fatigue (44%), rash (34%), decreased appetite (31%), musculoskeletal pain (27%), diarrhea/colitis (26%), dyspnea (26%), cough (23%), hepatitis (21%), nausea (21%), and pruritus (21%). In Checkmate 9LA, the most common (>20%) adverse reactions were fatigue (49%), musculoskeletal pain (39%), nausea (32%), diarrhea (31%), rash (30%), decreased appetite (28%), constipation (21%), and pruritus (21%).
Please see U.S. Full Prescribing Information for OPDIVO and YERVOY:
• https://packageinserts.bms.com/pi/pi_opdivo.pdf
• https://packageinserts.bms.com/pi/pi_yervoy.pdf
References:
1. Key statistics for lung cancer. American Cancer Society. Reviewed October 1, 2019. Revised January 8, 2020. Accessed October 7, 2020. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html.
2. Lung and bronchus cancer – cancer stat facts. National Cancer Institute. Accessed October 7, 2020. https://seer.cancer.gov/statfacts/html/lungb.html.
3. Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383:640-649.
4. OPDIVO [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
5. FDA approval for Checkmate 227. Accessed October 12, 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-nivolumab-plus-ipilimumab-first-line-mnsclc-pd-l1-tumor-expression-1.
6. YERVOY [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
7. FDA approval for Checkmate 9LA. Accessed October 12, 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-nivolumab-plus-ipilimumab-and-chemotherapy-first-line-treatment-metastatic-nsclc.
8. Weber JS, Hamid O, Chasalow SD, et al. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother. 2012;35:89-97.
9. Farber DL, Yudanin NA, and Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14(1):24-35.
10. Ansell SM, Hurvitz SA, Koenig PA, et al. Phase I study of ipilimumab, an anti–CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non–Hodgkin lymphoma. Clin Cancer Res. 2009;15(20):6446-6453.
11. Felix J, Lambert J, Roelens M, et al. Ipilimumab reshapes T cell memory subsets in melanoma patients with clinical response. Oncoimmunology. 2016;5(7):e1136045.
12. Pedicord VA, Montalvo W, Leiner IM, and Allison JP. Single dose of anti–CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci USA. 2011;108(1):266-271.
13. de Coaña YP, Wolodarski M, Poschke I, et al. Ipilimumab treatment decreases monocytic MDSCs and increases CD8 effector memory T cells in long-term survivors with advanced melanoma. Oncotarget. 2017;8(13):21539-21553.
14. Buchbinder EI and Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98-106.
15. Ramalingam S, Ciuleanu T-E, Pluzanski A, et al. Nivolumab + ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: Three-year update from Checkmate 227 Part 1. Oral presentation at ASCO 2020. Abstract 9500.
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381:2020-2031.
17. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381:2020-2031. [supplementary appendix].
18. Reck M, Ciuleanu T-E, Cobo M, et al. Nivolumab + ipilimumab + 2 cycles of platinum-doublet chemotherapy vs 4 cycles chemotherapy as first-line treatment for stage IV/recurrent NSCLC: Checkmate 9LA. Oral presentation at ASCO 2020. Abstract 9501.
19. Data on file. NIVO 566. Princeton, NJ: Bristol-Myers Squibb Company.

 and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2020, about 228, 820 new cases of lung cancer will be diagnosed and 135,720 patients will die of the disease.
and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2020, about 228, 820 new cases of lung cancer will be diagnosed and 135,720 patients will die of the disease.