SUMMARY: The FDA on May 27, 2022, granted accelerated approval to KYMRIAH® (Tisagenlecleucel) for adult patients with Relapsed or Refractory Follicular Lymphoma after two or more lines of systemic therapy. The American Cancer Society estimates that in 2022, about 80,470 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 20,250 individuals will die of this disease. Indolent Non Hodgkin Lymphomas are mature B cell lymphoproliferative disorders and include Follicular Lymphoma, Nodal Marginal Zone Lymphoma (NMZL), Extranodal Marginal Zone Lymphoma (ENMZL) of Mucosa-Associated Lymphoid Tissue (MALT), Splenic Marginal Zone Lymphoma (SMZL), LymphoPlasmacytic Lymphoma (LPL) and Small Lymphocytic Lymphoma (SLL). Follicular Lymphoma is the most indolent form and second most common form of all NHLs and they are a heterogeneous group of lymphoproliferative malignancies. Approximately 22% of all NHLs are Follicular Lymphomas (FL).
Advanced stage indolent NHL is not curable and as such, prolonging Progression Free Survival (PFS) and Overall Survival (OS), while maintaining Quality of Life, have been the goals of treatment intervention. Asymptomatic patients with indolent NHL are generally considered candidates for “watch and wait” approach. Patients with advanced stage symptomatic Follicular Lymphoma are often treated with induction chemoimmunotherapy followed by maintenance RITUXAN® (Rituximab). This can result in a median Progression Free Survival of 6-8 years. However, approximately 30% of the patients will relapse in 3 years and treatment options are limited for patients with relapses, after multiple treatments. Patients with Follicular Lymphomas often experience a relapsing and remitting pattern of disease and may be exposed to multiple lines of therapy over the course of their disease. In spite of the availability of multiple systemic therapies for Follicular Lymphoma, the efficacy of these regimens drops off rapidly with later lines of therapy. Novel therapies are therefore being investigated to improve outcomes.
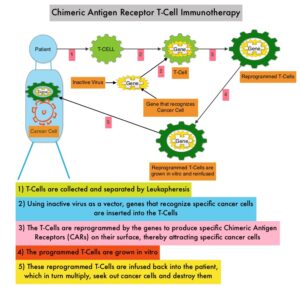
Chimeric Antigen Receptor (CAR) T-cell therapy is a type of immunotherapy and consists of T cells collected from the patient’s blood in a leukapheresis procedure, and genetically engineered to produce special receptors on their surface called Chimeric Antigen Receptors (CAR). These reprogrammed cytotoxic T cells with the Chimeric Antigen Receptors on their surface are now able to recognize a specific antigen on tumor cells. These genetically engineered and reprogrammed CAR T-cells are grown in the lab and are then infused into the patient. These cells in turn proliferate in the patient’s body and the engineered receptor on the cell surface help recognize and kill cancer cells that expresses that specific antigen. KYMRIAH® (genetically engineered T-cells) seeks out cancer cells expressing the antigen CD19, which is found uniquely on B cells and destroy them. Patients, following treatment with CAR T-cells, develop B-cell aplasia (absence of CD19 positive cells) due to B-cell destruction and may need immunoglobin replacement. Hence, B-cell aplasia can be a useful therapeutic marker, as continued B-cell aplasia has been seen in all patients who had sustained remission, following CAR T-cell therapy. Cytokine Release Syndrome, an inflammatory process is the most common and serious side effect of CAR T-cell therapy and is associated with marked elevation of Interleukin-6. Cytokine release is important for T-cell activation and can result in high fevers and myalgias. This is usually self limiting although if severe can be associated with hypotension and respiratory insufficiency. Tocilizumab (ACTEMRA®), an Interleukin-6 receptor blocking antibody produces a rapid improvement in symptoms. This is however not recommended unless the symptoms are severe and life threatening, as blunting the cytokine response can in turn negate T-cell proliferation. Elevated serum ferritin and C-reactive protein levels are surrogate markers for severe Cytokine Release Syndrome. The CAR T-cells have been shown to also access sanctuary sites such as the CNS and eradicate cancer cells. CD19 antigen is expressed by majority of the B-cell malignancies and therefore most studies using CAR T-cell therapy have focused on the treatment of advanced B-cell malignancies.
The present FDA approval was based on the ELARA trial, which is an international, multicenter, single-arm, open-label trial in which the efficacy and safety of KYMRIAH® was investigated in adult patients with Relapsed/Refractory Follicular Lymphoma, after at least two prior therapies. A total of 97 patients received KYMRIAH® (0.6-6×108 CAR+ viable T cells) after lymphodepleting chemotherapy. Bridging therapy was permitted followed by disease assessment prior to KYMRIAH® infusion. Eligible patients had Grades 1-3A Relapsed/Refractory Follicular Lymphoma who had progressed on 2 or more lines of systemic therapy, (including an anti-CD20 antibody and an alkylating agent) or relapsed after Autologous hematopoietic Stem Cell Transplant. The median patient age was 57 years, 85% had Stage III-IV disease, 60% had a FLIPI score 3 or more, 65% had bulky disease, and 42% had LDH above the upper limit of normal. The median number of prior therapies was 4, 78% of patients were refractory to their last treatment and 60% progressed within 2 years of initial anti-CD20 based therapy. The Primary endpoint was Complete Response Rate (CRR) by central review per Lugano 2014 criteria. Secondary endpoints included Overall Response Rate (ORR), Duration of Response (DOR), Progression Free Survival (PFS), Overall Survival (OS), Safety, and cellular kinetics.
In the primary efficacy analysis, with a median follow up 10.6 months, the Overall Response Rate was 86% with a Complete Response Rate of 66%. The response rates were comparable among key high risk subgroups. The median Duration of Response was Not Reached, with 75% of responders still in response at 9 months. At a median follow up of 17 months, the response rates were maintained and the 12-month PFS was 67% and 9 month Duration of Response was 76%. For patients who had a Complete Response, the 12-month PFS was 86% and the estimated Duration of Response was 87%. Approximately 48% of patients experienced Cytokine Release Syndrome (CRS) within eight weeks of infusion, with no patients experiencing CRS of Grade 3 or higher.
It was concluded that after a median follow up of 17 months, KYMRIAH® demonstrated high Response Rates, as well as durable responses, with remarkable safety profile, thus providing a new treatment option for this difficult-to-treat patient group of patients with Relapsed or Refractory Follicular Lymphoma.
Efficacy of Tisagenlecleucel in Adult Patients (Pts) with High-Risk Relapsed/Refractory Follicular Lymphoma (r/r FL): Subgroup Analysis of the Phase II Elara Study. Thieblemont C, Dickinson M, Martinez-Lopez J, et al. Presented in an oral session at the 63rd American Society of Hematology Annual Meeting & Exposition (ASH) 2021:(Abstract #131).