SUMMARY: The Center for Disease Control and Prevention (CDC) estimates that approximately 1-2 per 1000 individuals develop Deep Vein Thrombosis (DVT)/Pulmonary Embolism (PE) each year in the United States, resulting in 60,000-100,000 deaths. Venous ThromboEmbolism (VTE) is the third leading cause of cardiovascular mortality, after myocardial infarction and stroke. Ambulatory cancer patients initiating chemotherapy are at varying risk for Venous Thromboembolism (VTE), which in turn can have a substantial effect on health care costs, with negative impact on quality of life.
Approximately 20% of cancer patients develop VTE and there is a two-fold increase in the risk of recurrent thrombosis in patients with cancer, compared with those without cancer. The high risk of recurrent VTE, as well as bleeding in this patient group, makes anticoagulant treatment challenging. Treatment with parenteral Low Molecular Weight Heparin (LMWH) preparations is often recommended for this patient group, based on efficacy data. LMWH accelerates the inhibition by Antithrombin of activated Factor X, in the conversion of Prothrombin to Thrombin. Parenteral LMWH however can be inconvenient and expensive, leading to premature discontinuation of treatment.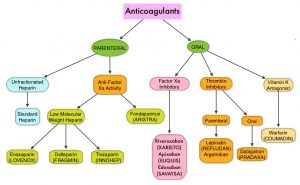
Direct Oral Anticoagulant agents have been proven to be as effective as COUMADIN® (Warfarin), a Vitamin K antagonist, for the treatment of VTE, and are associated with less frequent and less severe bleeding, and fewer drug interactions. The Direct Oral AntiCoagulants (DOACs) include PRADAXA® (Dabigatran), which is a direct Thrombin inhibitor and XARELTO® (Rivaroxaban), ELIQUIS® (Apixaban), SAVAYSA® (Edoxaban), BEVYXXA® (Betrixaban), which are Factor Xa inhibitors. Compared to COUMADIN®, the New Oral Anticoagulants have a rapid onset of action, wider therapeutic window, shorter half-lives (7-14 hours in healthy individuals), require no laboratory monitoring and have a fixed dosing schedule.
Three open-label, randomized, controlled trials have compared direct Factor Xa inhibitors with subcutaneous LMWH FRAGMIN® (Dalteparin). In the Hokusai VTE Cancer noninferiority trial, SAVAYSA® (Edoxaban) when compared with FRAGMIN® was associated with a lower rate of recurrent VTE, but this was offset by a similar increase in the risk of major bleeding. In the SELECT-D trial, XARELTO® was associated with relatively low VTE recurrence in patients with cancer, but with higher clinically relevant non-major bleeding, compared with FRAGMIN®. In the ADAM VTE Trial, oral ELIQUIS® therapy was associated with very low rates of bleeding and significantly lower VTE recurrence compared to parenteral FRAGMIN®. The inconsistent results from these studies have been attributed to patient selection (cancer type, types of cancer therapies and prognosis), duration of treatment, and primary efficacy outcomes of these studies. SAVAYSA® and XARELTO® are often recommended as alternatives to LMWH in patients with cancer, although higher risk of clinically important bleeding has been reported with both these agents, particularly in patients with GI malignancies, including pancreatic cancer.
The Caravaggio trial is a multinational, randomized, open-label, noninferiority trial which was conducted to assess whether oral ELIQUIS® would be noninferior to subcutaneous FRAGMIN® (Dalteparin), a LMWH, for the prevention of recurrent VTE in patients with cancer, without increasing the risk of major bleeding. In this study, 1155 patients with cancer who had symptomatic or incidental acute proximal DVT or PE were randomly assigned to receive ELIQUIS® 10 mg orally twice daily for the first 7 days, followed by 5 mg orally twice daily (N=576) or FRAGMIN® 200 IU/kg administered subcutaneously once daily for the first month, followed by 150 IU/kg subcutaneous once daily (N=579). The demographic and clinical characteristics of the patients in both treatment groups were well balanced and advanced active cancers associated with high thromboembolic risk such as lung and colorectal cancers were well represented. This study included patients receiving a variety of cytotoxic and biologic therapies. Anticoagulant treatments were administered for 6 months. The Primary endpoint was objectively confirmed recurrent VTE during the trial period. The principal safety outcome was major bleeding.
The Primary endpoint of recurrent VTE occurred in 5.6% of patients in the ELIQUIS® group and in 7.9% of patients in the FRAGMIN® group (HR=0.63; P<0.001 for noninferiority). Major bleeding occurred in 3.8% of patients in the ELIQUIS® group and 4.0% of patients in the FRAGMIN® group (HR=0.82; P=0.60). Major GI bleeding occurred in 1.9% of patients in the ELIQUIS® group and in 1.7% of patients in the FRAGMIN® group and major non-gastrointestinal bleeding occurred in 1.9% and 2.2% of patients respectively. There were no fatal bleeding episodes noted in the ELIQUIS® group, whereas 2 patients had a fatal bleed in the FRAGMIN® group. These findings with regards to bleeding are in contrast to the results of previously published studies, which showed a higher incidence of bleeding with other Direct Oral AntiCoagulants, compared with FRAGMIN®, in a similar patient population.
It was concluded that in this study which included patients with predominantly advanced active cancer and acute symptomatic VTE, oral ELIQUIS® was noninferior to subcutaneous FRAGMIN® for the treatment of cancer-associated VTE, without an increased risk of major bleeding. The authors added that these findings may expand the proportion of patients with both cancer and VTE who would be eligible for treatment with ELIQUIS®, including patients with active gastrointestinal malignancies. It should be noted however that LMWH should still be preferred for patients who have undergone surgery involving the upper GI tract, as Direct Oral AntiCoagulants are absorbed in the stomach or proximal small bowel, as well as for those patients with bleeding or thrombocytopenia, recurrent VTE, CNS cancers, or those with severe renal impairment, and in the perioperative setting.
Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. Agnelli G, Becattini C, Meyer G, et al. for the Caravaggio Investigators. N Engl J Med 2020; 382:1599-1607

