SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2020, about 228, 820 new cases of lung cancer will be diagnosed and 135,720 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of NSCLC, 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large Cell Carcinomas. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, Adenocarcinoma now is the most frequent histologic subtype of lung cancer.
The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. HER2 is a Tyrosine Kinase Receptor expressed on the surface of several tumor types including breast, gastric, lung and colorectal cancers. It is a growth-promoting protein and HER2 overexpression/HER2 gene amplification is often associated with aggressive disease and poor prognosis in certain tumor types. Other HER2 gene alterations such as HER2 mutations, as distinct molecular targets, have been identified in 2-4% of patients with NSCLC, specifically with adenocarcinoma histology. These acquired HER2 gene mutations have been independently associated with cancer cell growth and poor prognosis. There are currently no therapies approved specifically for the treatment HER2 mutant NSCLC, and is therefore an unmet need.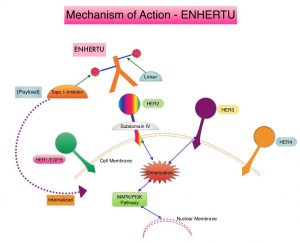
ENHERTU® (Trastuzumab Deruxtecan) is an Antibody-Drug Conjugate (ADC) composed of a humanized monoclonal antibody specifically targeting HER2, with the amino acid sequence similar to HERCEPTIN® (Trastuzumab), attached to a potent cytotoxic Topoisomerase I inhibitor payload by a cleavable tetrapeptide-based linker. ENHERTU® has a favorable pharmacokinetic profile and the tetrapeptide-based linker is stable in the plasma and is selectively cleaved by cathepsins that are up-regulated in tumor cells. Unlike KADCYLA® (ado-Trastuzumab emtansine), which is also an Antibody-Drug Conjugate, ENHERTU® has a higher drug-to-antibody ratio (8 versus 4), the released payload easily crosses the cell membrane with resulting potent cytotoxic effect on neighboring tumor cells regardless of target expression, and the released cytotoxic agent (payload) has a short half-life, minimizing systemic exposure. ENHERTU® is approved in the US for the treatment of adult patients with unresectable or metastatic HER2 positive breast cancer who received two or more prior anti-HER2 based regimens, based on the DESTINY-Breast01 trial.
DESTINY-Lung01 is an ongoing, global, multicenter, open-label, two-cohort, Phase II study, evaluating the safety and efficacy of ENHERTU® in 170 patients with HER2 mutant or HER2 overexpressing (defined as ImmunoHistoChemistry-IHC 3+ or IHC 2+), unresectable and metastatic non-squamous NSCLC. Eligible patients could not have received prior HER2-targeted therapy, with the exception of pan-HER TKIs. Patients were enrolled into 2 cohorts. The first cohort enrolled patients with HER2-expressing tumors as defined by IHC 3+ or 2+ (N = 42). The second cohort included patients whose tumors harbored a HER2 mutation as determined by a local laboratory test (N = 42). Enrolled patients had a median of two prior lines of therapy with majority of patients receiving platinum-based chemotherapy (90.5%), anti-PD-1 or PD-L1 treatment (54.8%) and 19% receiving Docetaxel. Patients received ENHERTU® 4.6 mg/kg every 3 weeks by intravenous infusion. The 42 patients included in the second cohort had a median age of 63 years, 64.3% of patients were female, and 45.2% had CNS metastases. For the majority of patients (90.5%), HER2 mutation was located in the kinase domain. The Primary endpoint was confirmed Objective Response Rate (ORR). Additional endpoints included Disease Control Rate (DCR), Duration of Response (DoR), Progression Free Survival (PFS), and safety. The authors reported data for the cohort with HER2 mutations (second cohort), after a median follow up of 8.0 months.
The ORR was 61.9%, with 2.4% Complete Response, 59.5% Partial Response, and stable disease noted in 28.6% of patients. The Disease Control Rate was 90.5%. The median PFS was 14 months. The median Duration of Response and Overall Survival (OS) had not yet been reached at the time of data cut-off. The most common Grade 3 or higher treatment related Adverse Events were neutropenia and anemia. Confirmed treatment-related Interstitial Lung Disease (ILD) and pneumonitis were noted in approximately 12% of patients and were all Grade 2 and there were no deaths. Nonetheless, ILD is an important identified risk for patients treated with ENHERTU® and requires careful monitoring and management.
It was concluded that ENHERTU® demonstrated promising clinical activity in this interim analysis, with a high Objective Response Rate and durable responses, in a heavily pretreated population of patients with HER2-mutated NSCLC.
Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-mutated metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINY-Lung01. Smit EF, Nakagawa K, Nagasaka M, et al. J Clin Oncol 38: 2020 (suppl; abstr 9504).

