SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 284,200 new cases of breast cancer will be diagnosed in 2021 and about 44,130 individuals will die of the disease, largely due to metastatic recurrence. Approximately 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors. The most common subtype of metastatic breast cancer is Hormone Receptor-positive (HR-positive), HER2-negative breast cancer (65% of all metastatic breast tumors), and these patients are often treated with anti-estrogen therapy as first line treatment. However, resistance to hormonal therapy occurs in a majority of the patients, with a median Overall Survival (OS) of 36 months. Cyclin Dependent Kinases (CDK) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies.
Cyclin Dependent Kinases 4 and 6 (CDK4 and CDK6) phosphorylate RetinoBlastoma protein (RB), and initiate transition from the G1 phase to the S phase of the cell cycle. RetinoBlastoma protein has antiproliferative and tumor-suppressor activity and phosphorylation of RB protein nullifies its beneficial activities. CDK4 and CDK6 are activated in hormone receptor positive breast cancer, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation. The understanding of the role of Cyclin Dependent Kinases in the cell cycle, has paved the way for the development of CDK inhibitors.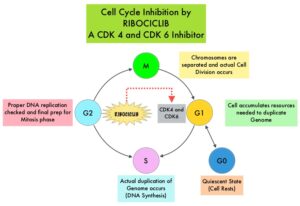
KISQALI® (Ribociclib) is an orally bioavailable, selective, small-molecule inhibitor of CDK4/6, that blocks the phosphorylation of RetinoBlastoma protein, thereby preventing cell-cycle progression and inducing G1 phase arrest.
In the MONALEESA-2 Phase III trial, KISQALI® in combination with FEMARA® (Letrozole) was compared to FEMARA® alone, in postmenopausal women with HR-positive, HER2-negative advanced breast cancer, who received no prior therapy for their advanced breast cancer. The addition of KISQALI® to FEMARA® significantly prolonged Progression Free Survival (PFS), compared to FEMARA® alone. In the MONALEESA-7 Phase III study, KISQALI® in combination with Tamoxifen or a Non-Steroidal Aromatase Inhibitor plus ZOLADEX® (Goserelin) was compared with placebo in combination with Tamoxifen or an Aromatase Inhibitor plus ZOLADEX®, in premenopausal or perimenopausal women with HR-positive, HER2- negative advanced breast cancer, who had not previously received endocrine therapy for advanced disease. In this study of premenopausal women, KISQALI® plus endocrine therapy significantly improved PFS and OS, compared with placebo plus endocrine therapy. The MONALEESA-7 trial recently reported an exploratory updated OS analysis with a median follow up of 53.5 months. In this analysis, KISQALI® plus endocrine therapy showed a clinically relevant and significant median OS benefit of 58.7 months compared with 48.0 months in the placebo plus endocrine therapy group.
MONALEESA-3 is a multicenter, international, randomized, double-blind, placebo-controlled Phase III study which compared the efficacy of KISQALI® in combination with FASLODEX® with FASLODEX® alone, among postmenopausal women with HR-positive, HER2-negative advanced breast cancer, who received no prior endocrine therapy or only one line of prior endocrine therapy for advanced disease. In this trial, 726 women were randomized, of whom 367 were treatment-naïve and 345 patients had received up to one line of prior endocrine therapy for advanced disease. Patients were randomized 2:1 to receive KISQALI® plus FASLODEX® (N=484) or placebo plus FASLODEX® (N=242). Treatment consisted of KISQALI® 600 mg orally daily 3 weeks on and 1 week off and FASLODEX® 500 mg IM on day 1 of each 28-day cycle, with an additional dose given on day 15 of cycle 1. Patients were stratified by the presence or absence of lung or liver metastases and prior endocrine therapy (first line versus second line). The median age in both groups was 63 years. The Primary endpoint was Progression Free Survival (PFS). Secondary end points included Overall Survival (OS), Overall Response Rate (ORR), and Safety. The authors had previously reported that in the MONALEESA-3 trial, there was a significant OS benefit for KISQALI® plus FASLODEX® versus placebo plus FASLODEX®, with an median OS of Not Reached (NR) versus 40.0 months (HR=0.72; P=0.00455).
The researchers in this publication reported an exploratory OS analysis update for MONALEESA-3, with an extended follow up (median, 56.3 months) in order to analyze the long-term OS benefit of KISQALI® plus FASLODEX® versus placebo plus FASLODEX®, similar to MONALEESA-7 trial. There was a significant OS benefit with a median OS of 53.7 months in the KISQALI® group versus 41.5 months in the placebo group (HR=0.73). The estimated 4-year survival rates were 54% and 45% for KISQALI® and placebo, respectively, while the 5-year survival rates were estimated to be 46% and 31% respectively. Subgroup analysis according to prior lines of endocrine therapy showed that patients in the first line subgroup had an median OS of Not Reached in the KISQALI® group and 51.8 months in the placebo group (HR=0.64). In the second line setting, the median OS was 39.7 months in the KISQALI® group versus 33.7 months in the placebo group (HR=0.78). No new safety signals were observed.
It was concluded that this extended follow up analysis of MONALEESA-3 is the longest reported follow up for any CDK4/6 inhibitor clinical trial in the postmenopausal women, and demonstrated a durable Overall Survival benefit with KISQALI® plus FASLODEX®, compared to FASLODEX® alone, in patients with HR-positive, HER2-negative advanced breast cancer. Further, this benefit was maintained when KISQALI® was given both as first line as well as second line therapy, and across subgroups of patients studied.
Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Slamon DJ, Neven P, Chia S, et al. Annals of Oncology 2021; 32:1015-1024.

