Approximately 35-40% of ovarian cancer patients express high levels of Folate Receptor alpha, and this expression correlates with advanced stages of disease and more malignant phenotypes. There is limited expression of Folate Receptor alpha in normal tissues and is limited to the choroid plexus, proximal renal tubules, placenta, and endometrium. Testing for Folate Receptor alpha can be performed on fresh or archived tissue and is considered positive if at least 75% of cells had 2+ staining intensity or greater, based on immunohistochemistry-based scoring. .
Author: RR
ESR1 Gene Mutation
ESR1 (Estrogen Receptor 1) gene mutation is the most common acquired mutation noted in breast tumors as they progress from primary to metastatic setting. These mutations promote ligand independent Estrogen Receptor activation and have been shown to promote resistance to estrogen deprivation therapy. It appears that ESR1 mutations are harbored in metastatic ER-positive breast cancers treated with prior Aromatase Inhibitor (AI) therapy in the metastatic setting, but not in primary breast cancers, suggesting that ESR1 mutations may be selected by prior therapy with an AI in advanced breast cancer. Approximately 40% of patients who had received AI therapy for metastatic breast cancer have ESR1 mutations. It is best to test for ESR1 mutations with a liquid biopsy following progression on an AI and CDK 4/6 inhibitor.
Overall Survival Benefit with Pembrolizumab in Advanced Gastric Cancer
SUMMARY: The American Cancer Society estimates that in the US about 26,500 new gastric cancer cases will be diagnosed in 2023 and about 11,130 people will die of the disease. It is one of the leading causes of cancer-related deaths in the world. Several hereditary syndromes such as Hereditary Diffuse Gastric Cancer (HDGC), Lynch syndrome (Hereditary Nonpolyposis Colorectal Cancer) and Familial Adenomatous Polyposis (FAP) have been associated with a predisposition for Gastric cancer. Additionally, one of the strongest risk factor for Gastric adenocarcinoma is infection with Helicobacter pylori (H.pylori), which is a gram-negative, spiral-shaped microaerophilic bacterium.
Patients with localized disease (Stage II and Stage III) are often treated with multimodality therapy and 40% of the patients may survive for 5 years or more. However, majority of the patients with Gastric and GastroEsophageal junction Adenocarcinoma have advanced disease at the time of initial presentation and have limited therapeutic options with little or no chance for cure. These patients frequently are treated with Platinum containing chemotherapy along with a Fluoropyrimidine and, if appropriate, HER2/neu-targeted therapy. This can however be associated with significant toxicities impacting patient’s quality of life. The efficacy of PD-1 inhibitors in combination with chemotherapy has been demonstrated in Gastric and GastroEsophageal cancer.
KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. It thereby reverses the PD-1 pathway-mediated inhibition of the immune response and unleashes the tumor-specific effector T cells.
KEYNOTE-859 was a double-blind, placebo-controlled, randomized Phase III trial, conducted to evaluate the benefit of adding Pembrolizumab to Fluoropyrimidine and Platinum-containing doublet chemotherapy in patients with advanced HER2-negative Gastric or GastroEsophageal cancer. In this study, 1,579 patients with locally advanced or metastatic HER2-negative Gastric or GastroEsophageal adenocarcinoma, with known a PD-L1 Combined Positive Score (CPS), were randomly assigned 1:1 to receive Pembrolizumab 200 mg IV (N=790) or placebo (N=789), every 3 weeks for 35 cycles or less, given along with investigator’s choice of 5-FU plus Cisplatin or Capecitabine plus Oxaliplatin (CAPOX). Baseline characteristics were balanced between treatment groups and randomization was stratified by region, PD-L1 CPS (less than 1 versus 1 or more), and choice of chemotherapy. At baseline, 78% of patients had PD-L1 CPS 1 or more, while 35% had tumors with CPS 10 or more.
The Primary end point was Overall Survival (OS) by blinded Independent Central Review. Secondary end points included Progression Free Survival (PFS), Objective Response Rate (ORR), Duration of Response (DOR) and Safety. The researchers provided the data from the interim analysis, at a median follow up of 31.0 months.
The median Overall Survival was 12.9 months with Pembrolizumab plus chemotherapy versus 11.5 months with chemotherapy alone (HR=0.78, P<0.0001). The median PFS was 6.9 months versus 5.6 months, respectively (HR=0.76, P<0.0001). The benefit with Pembrolizumab was consistent across subgroups, including those by PD-L1 CPS. The risk reduction was especially notable among patients with MicroSatellite Instability (MSI)-High status, who had a 66% relative reduction in the risk of death, and patients with PD-L1 CPS 10 or more, whose risk was reduced by 36%. The Objective Response Rate was 51.3% in the Pembrolizumab group and 42.0% in the control group (P=0.00009), and the median Duration of Response was 8.0 months versus 5.7 months, respectively. Immune-related toxicities, especially hypothyroidism, were more common with Pembrolizumab plus chemotherapy and no new safety signals were seen.
It was concluded that treatment with Pembrolizumab plus chemotherapy resulted in a statistically significant and clinically meaningful improvement in Overall Survival, Progression Free Survival and Objective Response Rate, among patients with locally advanced or metastatic, HER2-negative Gastric or GastroEsophageal adenocarcinoma of any PD-L1 expression level, thus providing a new treatment option for this patient group.
Pembrolizumab plus chemotherapy as first-line therapy for advanced HER2-negative gastric or gastroesophageal junction cancer: Phase III KEYNOTE-859 study. Rha SY, Wyrwicz LS, Weber PEY, et al. ESMO Virtual Plenary Session Date: 16-17 February 2023. VP1-2023. Published: February 16, 2023. DOI: https://doi.org/10.1016/j.annonc.2023.01.006.
Rucaparib or Physicians Choice of Therapy in Metastatic Prostate Cancer
SUMMARY: Prostate cancer is the most common cancer in American men with the exclusion of skin cancer, and 1 in 9 men will be diagnosed with Prostate cancer during their lifetime. It is estimated that in the United States, about 288,300 new cases of Prostate cancer will be diagnosed in 2023 and 34,700 men will die of the disease.
The development and progression of Prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) or testosterone suppression has therefore been the cornerstone of treatment of advanced Prostate cancer and is the first treatment intervention. Androgen Deprivation Therapies have included bilateral orchiectomy or Gonadotropin Releasing Hormone (GnRH) analogues, with or without first generation Androgen Receptor (AR) inhibitors such as CASODEX® (Bicalutamide), NILANDRON® (Nilutamide) and EULEXIN® (Flutamide) or with second-generation Androgen-Receptor Pathway Inhibitors (ARPI), which include, ZYTIGA® (Abiraterone), XTANDI® (Enzalutamide) and ERLEADA® (Apalutamide). Approximately 10-20% of patients with advanced Prostate cancer will progress to Castration Resistant Prostate Cancer (CRPC) within five years during ADT, and over 80% of these patients will have metastatic disease at the time of CRPC diagnosis. The estimated mean survival of patients with CRPC is 9-36 months, and there is therefore an unmet need for new effective therapies.
DNA damage is a common occurrence in daily life by UV light, ionizing radiation, replication errors, chemical agents, etc. This can result in single and double strand breaks in the DNA structure which must be repaired for cell survival. The two vital pathways for DNA repair in a normal cell are BRCA1/BRCA2 and PARP. BRCA1 and BRCA2 genes recognize and repair double strand DNA breaks via Homologous Recombination Repair (HRR) pathway. Homologous Recombination is a type of genetic recombination and is a DNA repair pathway utilized by cells to accurately repair DNA double-stranded breaks during the S and G2 phases of the cell cycle, and thereby maintain genomic integrity. Homologous Recombination Deficiency (HRD) is noted following mutation of genes involved in HR repair pathway. At least 15 genes are involved in the Homologous Recombination Repair (HRR) pathway including BRCA1, BRCA2 and ATM genes. The BRCA1 gene is located on the long (q) arm of chromosome 17 whereas BRCA2 is located on the long arm of chromosome 13. BRCA1 and BRCA2 are tumor suppressor genes and functional BRCA proteins repair damaged DNA, and play an important role in maintaining cellular genetic integrity. They regulate cell growth and prevent abnormal cell division and development of malignancy. Recently published data has shown that deleterious Germline and/or Somatic mutations in BRCA1, BRCA2, ATM, or other Homologous Recombination DNA-repair genes, are present in about 25% of patients with advanced prostate cancer, including metastatic CRPC. Approximately 12% of men with metastatic CRPC harbor a deleterious BRCA1 or BRCA2 mutation (BRCA1, 2%; BRCA2, 10%). Mutations in BRCA1 and BRCA2 also account for about 20-25% of hereditary breast cancers, about 5-10% of all breast cancers, and 15% of ovarian cancers.
The PARP (Poly ADP Ribose Polymerase), family of enzymes include, PARP1and PARP2, and is a related enzymatic pathway that repairs single strand breaks in DNA. In a BRCA mutant, the cancer cell relies solely on PARP pathway for DNA repair to survive. PARP inhibitors trap PARP onto DNA at sites of single-strand breaks, preventing their repair and generating double-strand breaks that cannot be repaired accurately in tumors harboring defects in Homologous Recombination Repair pathway genes, such as BRCA1 or BRCA2 mutations, and this leads to cumulative DNA damage and tumor cell death.
RUBRACA® (Rucaparib) is an oral, small molecule inhibitor of PARP inhibitor, and in the Phase II TRITON2 study, Rucaparib showed a high level of activity in metastatic Castration Resistant Prostate Cancer (CRPC) associated with a deleterious BRCA alteration, in patients who had received previous treatment with a second-generation Androgen-Receptor Pathway Inhibitor (ARPI) and taxane-based chemotherapy.
TRITON3 is an open-label, controlled, randomized, Phase III trial, conducted to evaluate the benefit of Rucaparib in men with metastatic CRPC at an earlier stage of treatment, and to confirm and expand on data from the TRITON2 study. This study enrolled patients who had metastatic CRPC with a BRCA1, BRCA2, or ATM alteration, who had disease progression after treatment with a second-generation ARPI, and who had not received previous chemotherapy for metastatic CRPC. Patients were randomly assigned in a 2:1 ratio to receive Rucaparib 600 mg orally twice daily or a physician’s choice of therapy (Docetaxel or a second-generation ARPI such as Abiraterone acetate or Enzalutamide). Abiraterone acetate or Enzalutamide could not be selected if the patient had received either drug before trial initiation. Approximately 56% received Docetaxel in the control group. The median age was 70 years and baseline genomic, demographic, and disease characteristics were well balanced in the two groups although men of African descent were underrepresented relative to the general population. Among the patients who had undergone randomization, 302 patients had a BRCA alteration and 103 patients had an ATM alteration. In this study, there were smaller numbers of patients with BRCA1 alterations than with BRCA2 alterations. The Primary end point was the median duration of imaging-based Progression Free Survival (PFS) according to Independent Review. Secondary outcomes included Overall Survival (OS) and Objective Response Rate (ORR), Duration of Response, Time to progression according to Prostate Specific Antigen (PSA) testing and Patient-Reported Outcomes.
At 62 months, the median duration of imaging-based PFS was significantly longer in the Rucaparib group than in the control group, both in the BRCA subgroup (11.2 months and 6.4 months, respectively; HR=0.50) and in the intention-to-treat group (10.2 months and 6.4 months, respectively; HR=0.61; P<0.001 for both comparisons). These findings demonstrating the benefit of Rucaparib compared to the Docetaxel control group are significant, as numerous previous studies either did not include Docetaxel in the control group, or did not show the superiority of PARP inhibition to Docetaxel. These study findings were consistent with the results of previous studies, suggesting that repeated use of second-generation ARPIs appeared to have only modest activity and inferior to PARP inhibition. Among patients with measurable disease at baseline, the confirmed Objective Response in the Rucaparib group and the control group was 45% and 17% respectively in the BRCA subgroup, 35% and 16% respectively, in the intention-to-treat population and no response and 14% respectively in the ATM subgroup. Because there were a smaller number of patients with BRCA1 alterations than with BRCA2 alterations in this study, the treatment benefit was not conclusive in those with BRCA1 alterations.
In an exploratory analysis in the ATM subgroup, the median duration of imaging-based PFS was 8.1 months in the Rucaparib group and 6.8 months in the control group (HR=0.95), suggesting limited efficacy of Rucaparib in the ATM subgroup, similar to the results of previous clinical trials involving PARP inhibitors. The most frequent adverse events with Rucaparib were fatigue and nausea.
It was concluded that in patients with metastatic Castration-Resistant Prostate Cancer in whom treatment with an Androgen Receptor Pathway Inhibitor (ARPI) had failed, the use of Rucaparib resulted in a longer duration of imaging-based Progression Free Survival than a physician’s choice of Docetaxel or a second-generation ARPI.
Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. Fizazi K, Piulats JM, Reaume MN, et al., for the TRITON3 Investigators. N Engl J Med 2023; 388:719-732.
TUKYSA® (Tucatinib)
The FDA on January 19, 2023, granted accelerated approval to TUKYSA® in combination with Trastuzumab for RAS wild-type HER2-positive unresectable or metastatic colorectal cancer that has progressed following Fluoropyrimidine, Oxaliplatin, and Irinotecan-based chemotherapy. TUKYSA® is a product of Seagen Inc.
LUNSUMIO® (Mosunetuzumab-axgb)
The FDA on December 22, 2022 granted accelerated approval to LUNSUMIO®, a bispecific CD20-directed CD3 T-cell engager for adult patients with Relapsed or Refractory Follicular Lymphoma (FL) after two or more lines of systemic therapy. LUNSUMIO® is a product of Genentech, Inc.
Breast-Conserving Surgery with or without Irradiation in Early Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. It is estimated that approximately 300,590 new cases of breast cancer will be diagnosed in 2023 and about 43,700 individuals will die of the disease, largely due to metastatic recurrence. About 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors, and Hormone Receptor (HR)-positive/HER2-negative breast cancer is the most frequently diagnosed molecular subtype. Majority of these patients are diagnosed with early stage disease and are often cured with a combination of surgery, radiotherapy, adjuvant chemotherapy, and hormone therapy. However approximately 20% of patients will experience local recurrence or distant relapse during the first 10 years of treatment.
The median age at the time of breast cancer diagnosis in the US is 62 years and approximately 26% of breast cancer diagnoses are in women 65 to 74 years of age. Patient undergoing breast conserving surgery, often receive adjuvant breast radiation therapy to reduce the risk of local recurrence. Radiation therapy however is inconvenient, expensive and is associated with acute and late toxicities. Avoidance of radiation in elderly patients with low risk disease has remained controversial due to the lack of long term Level 1 evidence. In the LUMINA trial and PRIME II study of women over 55 years of age with low risk breast cancer, after a median follow up of 5 years, 5-year rate of ipsilateral breast tumor recurrence was low at 2-4% among those women who did not receive adjuvant whole-breast radiotherapy after breast-conserving surgery. The researchers herein reported the 10-year outcomes of the PRIME II trial.
PRIME II is a Phase III randomized clinical trial of the omission of breast irradiation, designed by the Scottish Cancer Trials Breast Group (SCTBG). This study included women 65 years of age or older, who had Hormone Receptor (HR)-positive, node-negative, T1 or T2 primary breast cancer (with tumors 3 cm or less in the largest dimension), treated with breast-conserving surgery with clear excision margins and adjuvant endocrine therapy. Patients were eligible if they had either cancer with Grade 3 histologic features or lymphovascular invasion but not both. A total of 1326 women were randomly assigned to receive 40-50 Gy whole-breast irradiation (N=658) or no radiation therapy (N=668). Both treatment groups were well balanced. The median patient age was 70 years and less than 10% of patients had ER-low tumors. The Primary end point was local breast cancer recurrence. Regional recurrence, breast cancer–specific survival, distant recurrence as the first event, and Overall Survival were also assessed. The median follow up was 9.1 years.
The cumulative incidence of local breast cancer recurrence after 10 years of follow up was 9.5% in the no-radiotherapy group and 0.9% in the radiotherapy group (HR=10.4; P<0.001). Even though local recurrence was more common in the group that did not receive radiotherapy, there was no substantial difference in the 10-year incidence of distant recurrence as the first event between the two treatment groups (1.6% without radiotherapy and 3.0% with radiotherapy). Overall Survival at 10 years was almost identical in the two groups, at 80.8% with no radiotherapy and 80.7% with radiotherapy. The incidence of regional recurrence and breast cancer–specific survival also did not differ substantially between the two groups.
The authors concluded that omission of radiotherapy was associated with an increased incidence of local recurrence but had no detrimental effect on distant recurrence as the first event, or Overall Survival, among women 65 years of age or older, with Grade 1 or 2, Estrogen Receptor-high breast cancers, treated with breast-conserving surgery and 5 years of adjuvant endocrine therapy.
Breast-Conserving Surgery with or without Irradiation in Early Breast Cancer. Kunkler IH, Williams LJ, Jack WJL, et al. N Engl J Med 2023; 388:585-594.
Lobar or Sublobar Resection for Peripheral Stage IA Non Small Cell Lung Cancer
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 21% of all cancer deaths. The American Cancer Society estimates that for 2023, about 238,340 new cases of lung cancer will be diagnosed and 127,070 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of NSCLC, 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large Cell Carcinomas. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, Adenocarcinoma now is the most frequent histologic subtype of lung cancer.
Low Dose CT (LDCT) screening for lung cancer resulted in a 20% reduction in mortality In the National Lung Screening Trial (NLST). The USPSTF expanded the criteria for lung cancer screening in 2021 and recommended annual screening with Low-Dose CT for adults aged 50-80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years. Approximately 15% of patients present with early stage (T1-2 N0) disease, and these numbers are likely to increase with the more rigorous implementation of lung cancer screening programs.
Surgical resection is the primary treatment for approximately 30% of patients with NSCLC who present with early Stage (I–IIIA) disease. Pneumonectomy is rarely performed due to unacceptably high mortality rate. Lobectomy has been the standard of surgical care for patients with clinical T1N0 NSCLC since the mid 1990s. This was based on the results of a randomized trial comparing Lobectomy with Sublobar resection in patients with clinical T1N0 NSCLC. In this trial, the frequency of local recurrence was three times higher with Sublobar resection compared with Lobectomy, and lung cancer-related mortality was 50% higher with Sublobar resection.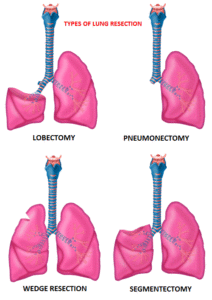
Sublobar resection includes Wedge resection and Segmentectomy. In Wedge resection, the lung tumor is removed with a surrounding margin of normal lung tissue, and is not an anatomical resection. Segmentectomy, unlike Wedge resection, is an anatomical resection that usually includes one or more pulmonary parenchymal segments with the dissection of intraparenchymal and hilar lymph nodes. Advances in imaging as well as staging by means of mediastinoscopy, and routine intraoperative lymphadenectomy has enabled the identification of small, peripheral NSCLCs for which Sublobar resection was potentially appropriate. Sublobar resection was considered a “compromise operation” in selected high risk patients with early stage lung cancer. With the approval of lung cancer screening in high risk individuals and subsequent detection of small tumors, Sublobar resections have been on the rise and may be the preferred surgical option, even in good-risk patients, in many institutions. Sublobar resection preserves pulmonary function and leaves open more treatment options for NSCLC patients, who remain at high risk for metachronous primary NSCLC, following curative intervention for their first NSCLC.
With the implementation of CT-based lung cancer screening recently, lung cancers are likely detected at a very early stage (T1a-bN0; 2 cm or less, node negative tumors). Further, Adenocarcinoma now is the most frequent histologic subtype of lung cancer and present as peripherally located tumors. Advances in preoperative staging such as endobronchial ultrasonography, have improved patient selection for treatment. Majority of surgical resections are now performed by means of video or robotic-assisted thoracic surgery. This has improved postoperative outcomes, with significant reduction in perioperative morbidity, mortality and median length of hospital stay after either Sublobar resection or Lobectomy.
The authors in this study reported the results of a randomized international trial comparing Sublobar resection (wedge resection or segmentectomy) with Lobectomy, in patients with clinical Stage IA NSCLC, with a tumor size of 2 cm or less. Cancer and Leukemia Group B (CALGB) 140503 was a multicenter, international, randomized, noninferiority, Phase III trial, involving patients with NSCLC clinically staged as T1aN0. In this study, a total of 697 patients, after intraoperative confirmation of node-negative disease, were randomly assigned to undergo either Sublobar resection (N=340) or Lobar resection (N=357). Of the 340 patients assigned to Sublobar resection, 201 (59.1%) underwent wedge resection and 129 (37.9%) underwent an anatomical segmental resection. Wedge resection was allowed in the current trial as it is the most frequently practiced method of Sublobar resection in North America and Europe and its inclusion would make the trial more representative of a “real world” setting. The median patient age was 68 years. Approximately 50% of patients had tumor size 1.0-1.5 cm, 40% had tumor size 1.5-2.0 cm, and two thirds of the patients had Adenocarcinoma histology. Over 90% of the patients were current or former smokers. The Primary end point was Disease-Free Survival (DFS), defined as the time between randomization and disease recurrence or death from any cause. Secondary end points included Overall Survival (OS), locoregional and systemic recurrence, and pulmonary functions.
After a median follow up of 7 years, Sublobar resection was noninferior to Lobar resection for DFS (HR for disease recurrence or death=1.01). The 5-year DFS was 63.6% after Sublobar resection and 64.1% after Lobar resection. The Overall Survival after Sublobar resection was similar to that after Lobar resection (HR for death, 0.95). The 5-year OS was 80.3% after Sublobar resection and 78.9% after Lobar resection. No substantial difference was seen between the two groups in the incidence of locoregional or distant recurrence. At 6 months postoperatively, pulmonary functions favored the Sublobar resection group.
It was concluded that Sublobar resection by either anatomical segmentectomy or wedge resection, for patients with peripheral NSCLC with a tumor size of 2 cm or less and pathologically confirmed node-negative disease in the hilar and mediastinal lymph nodes, was non inferior to Lobectomy, with respect to Disease Free Survival and with similar Overall Survival, and is an effective management approach for this subgroup of patients with NSCLC.
Lobar or Sublobar Resection for Peripheral Stage IA Non–Small-Cell Lung Cancer. Altorki N, Wang X, Kozono D, et al. N Engl J Med 2023; 388:489-498
A POSITIVE Trial for Hormone Receptor Positive Breast Cancer Survivors Desiring Pregnancy
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. It is estimated that approximately 300,590 new cases of breast cancer will be diagnosed in 2023 and about 43,700 individuals will die of the disease, largely due to metastatic recurrence. About 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors, and Hormone Receptor (HR)-positive/HER2-negative breast cancer is the most frequently diagnosed molecular subtype. Majority of these patients are diagnosed with early stage disease and are often cured with a combination of surgery, radiotherapy, adjuvant chemotherapy, and hormone therapy. However approximately 20% of patients will experience local recurrence or distant relapse during the first 10 years of treatment.
The median age at the time of breast cancer diagnosis in the US is 62 years. However approximately 5% of new diagnoses each year occur in those who are under 40 years. These young patients with Hormone Receptor (HR)-positive breast cancer receiving modern adjuvant endocrine therapy have excellent long-term outcomes. Nonetheless, 40-60% of patients who are diagnosed with breast cancer at age 40 or younger are concerned about their future fertility and pregnancy, as many have not completed their family planning at diagnosis due to delay in childbearing.
The POSITIVE (Pregnancy Outcome and Safety of Interrupting Therapy for Women with Endocrine Responsive Breast Cancer) trial is a multicenter, global, single-arm prospective study, designed to evaluate whether temporary interruption of adjuvant endocrine therapy to attempt pregnancy is associated with a higher risk of breast cancer recurrence. This study included 517 women with Stage I-III Hormone Receptor (HR)-positive early breast cancer, 42 years or less, who had received 18-30 months of adjuvant endocrine therapy and wished to interrupt endocrine therapy for pregnancy. The study permitted treatment interruption for up to 2 years (after a 3 month endocrine therapy washout period) to allow pregnancy, delivery and breastfeeding, followed by endocrine therapy resumption to complete the planned duration of 5-10 of adjuvant endocrine therapy. The median age was 37 years, 75% were nulliparous, fertility preservation was used by 51% of women, 93% had Stage I/II disease, 66% were node negative and 62% had received neo/adjuvant chemotherapy. Tamoxifen alone was the most prescribed endocrine therapy (42%), followed by Tamoxifen plus Ovarian Function Suppression (OFS) (35%).
The Primary endpoint of the study was Breast Cancer-Free Interval (BCFI), defined as the time from study enrollment to the first invasive breast cancer event (local/regional/distant recurrence or contralateral breast cancer). Three interim safety analyses were conducted by a Data Safety Monitoring Committee, and determined that the trial would be suspended if there were more than 46 breast cancer recurrences within approximately 3 years of average follow-up. This threshold however was not reached.
At a median follow up of 41 months, of the 497 patients evaluated for pregnancy status, 74% (N=368) had at least one pregnancy, with 70% of the pregnancies occurring within 2 years. Additionally, 63.8% (N=317) had at least one live birth, with a total of 365 babies born. Birth defects were low at 2% and the rates of conception and childbirth were similar to rates in the general public. The 3-year breast cancer recurrence rate among patients who halted therapy was 8.9%, similar to the 9.2% rate in an external control cohort from the SOFT/TEXT trials, which examined adjuvant endocrine therapy in premenopausal patients. Long term follow up is ongoing to assess recurrence risk over time, and trial participants were strongly recommended to resume endocrine therapy following their pregnancy attempts or success.
The authors concluded that these data provide guidance to younger patients diagnosed with early breast cancer on endocrine therapy, who may be hoping to have children. They added that such decisions should be made in close consultation with health professionals.
Pregnancy outcome and safety of interrupting therapy for women with endocrine responsive breast cancer: primary results from the POSITIVE trial (IBCSG 48-14 / BIG 8-13). Partridge A, Niman SM, Ruggeri M, et al. Presented at 2022 San Antonio Breast Cancer Symposium; December 6-10, 2022; San Antonio, TX.
FDA Approves JAYPIRCA® for Relapsed or Refractory Mantle Cell Lymphoma
SUMMARY: The FDA on January 27, 2023, granted accelerated approval to JAYPIRCA® (Pirtobrutinib) for Relapsed or Refractory Mantle Cell Lymphoma (MCL) after at least two lines of systemic therapy, including a BTK inhibitor.
The American Cancer Society estimates that in 2023, about 80,550 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 20,180 individuals will die of this disease. In the US, approximately 3,300 new cases of MCL are diagnosed each year. Mantle Cell Lymphoma is an aggressive B-cell lymphoma and accounts for approximately 6% of all Non Hodgkin Lymphomas in adults, and is associated with a high relapse rate, following dose-intensive therapies. Early and late relapses in patients with MCL have been attributed to persistence of residual disease. Majority of patients with MCL are elderly and are not candidates for aggressive treatment or Autologous Stem Cell Transplantation.
Bruton’s Tyrosine Kinase (BTK) is a member of the Tec family of kinases, downstream of the B-cell receptor, and is predominantly expressed in B-cells. It is a mediator of B-cell receptor signaling in normal and transformed B-cells. BTK inhibitors inhibit cell proliferation and promotes programmed cell death (Apoptosis) by blocking B-cell activation and signaling. BTK is a validated molecular target found across numerous B-cell leukemias and lymphomas including Chronic Lymphocytic Leukemia (CLL), Mantle Cell Lymphoma (MCL), and Waldenstrom Macroglobulinemia (WM). The 3 covalent BTK inhibitors presently approved by the FDA for MCL include IMBRUVICA® (Ibrutinib) approved in 2013, CALQUENCE® (Acalabrutinib) approved in 2017, and BRUKINSA® (Zanubrutinib) approved in 2019. Covalent BTK inhibitors have transformed the treatment landscape of Mantle Cell Lymphoma. Despite the efficacy of covalent BTK inhibitors, treatment failure often occurs through development of resistance or intolerance.
JAYPIRCA® is a highly selective, reversible (non-covalent) Bruton’s Tyrosine Kinase (BTK) inhibitor, developed to reversibly bind BTK, deliver consistently high target coverage regardless of BTK turnover rate, and preserve activity in the presence of the C481 acquired resistance mutations. JAYPIRCA® is 300 times more selective in BTK inhibition versus 98% of other kinases tested in preclinical studies and inhibits both wildtype and C481-mutant BTK with equal low nM potency, and has favorable oral pharmacology. JAYPIRCA® is well tolerated and demonstrated promising efficacy in patients with poor-prognosis B-cell malignancies following prior therapy, including prior covalent BTK inhibitors (Mato et al. Lancet, 2021).
The BRUIN Phase I/II clinical trial is the ongoing first-in-human, global, open-label, multicenter single armstudy, which evaluated the efficacy of JAYPIRCA® in previously treated patients with Mantle Cell Lymphoma (MCL), Chronic Lymphocytic Leukemia (CLL), Small Lymphocytic Lymphoma (SLL), or other Non-Hodgkin Lymphomas (NHL). The trial included a Phase I dose-escalation component in which the daily dosing of JAYPIRCA® between 25 mg and 300 mg was evaluated, a Phase Ib drug combination safety arm, and a Phase II dose-expansion component, in which JAYPIRCA® 200 mg daily, as a part of 28-day cycles, is being evaluated.
The present FDA approval is based on data from a subset of patients with Mantle Cell Lymphoma (N=120) in the BRUIN Phase I/II trial, treated with JAYPIRCA® 200 mg once daily until disease progression or unacceptable toxicity. Patients had received a median of three prior lines of therapy with 93% having two or more prior lines, and all patients received one or more prior lines of therapy containing a covalent BTK inhibitor. The most common prior BTK inhibitors received were Ibrutinib (67%), Acalabrutinib (30%), and Zanubrutinib (8%), and 83% had discontinued their last BTK inhibitor due to refractory or progressive disease. Patients with active Central Nervous System Lymphoma or allogeneic Hematopoietic Stem Cell Transplantation or CAR T-cell therapy within 60 days were excluded. The main efficacy measures were Overall Response Rate (ORR) and Duration of Response (DOR), as assessed by an Independent Review Committee, using 2014 Lugano criteria.
The ORR was 50%, with a Complete Response rate of 13%, and the Partial Response rate was 38%. The Time to Response was 1.8 months. The estimated median Duration of Response was 8.3 months, and the estimated Duration of Response rate at 6 months was 65.3%. The most common adverse reactions in 15% or more of MCL patients were fatigue, musculoskeletal pain, diarrhea, edema, dyspnea, pneumonia, and bruising. Grade 3 or 4 laboratory abnormalities in 10% or more of patients were cytopenias.
It was concluded that JAYPIRCA® offers a new approach to targeting the BTK pathway for Relapsed and Refractory Mantle Cell Lymphoma patients, previously treated with a covalent BTK inhibitor. The researchers added that this approval of JAYPIRCA® represents an important advance for this group of patients who currently have limited treatment options and have a poor prognosis.
Efficacy of Pirtobrutinib in Covalent BTK-Inhibitor Pre-Treated Relapsed / Refractory Mantle Cell Lymphoma: Additional Patients and Extended Follow-up from the Phase 1/2 BRUIN Study. Wang ML, Shah NN, Jurczak W. Presented at the 64th ASH Annual Meeting and Exposition, December 10-13, 2022, New Orleans, Louisiana. Abstract # 4218.
