Written By: Leonel Fernando Hernandez Aya, MD. Division of Medical Oncology, Department of Medicine, University of Miami Miller School of Medicine, Sylvester Comprehensive Cancer Center
Content Sponsored by: Bristol Myers Squibb
Dr Hernandez Aya is a paid consultant for BMS and was compensated for his contribution in drafting this content.
See additional definitions of abbreviations used throughout the article at the bottom of this page.
Overview of Metastatic Melanoma
Since the approval of anti–CTLA-4 in 2011 for metastatic melanoma, immuno-oncology(I-O) has transformed treatment outcomes.1 There are now several approved I-O options, and of those approved for the treatment of metastatic melanoma, dual immunotherapy in particular has had long-term success.2 The first dual immunotherapy, approved in 2015, consisted of PD-1 and CTLA-4 checkpoint inhibitors for the 1L treatment of unresectable or metastatic melanoma, regardless of BRAF mutation status.1,3,4 This anti–PD-1 and anti–CTLA-4 combination showed benefit in overall survival (OS) compared with anti–CTLA-4 alone.5 In general, the safety profile was consistent with previous experience with anti–PD-1 or anti–CTLA-4 alone.4 Until March 2022, this dual anti–PD-1 and anti–CTLA-4 immunotherapy was the only option indicated for the 1L treatment of unresectable or metastatic melanoma.3,6 Opdualag, the second approved dual immunotherapy, has provided an additional treatment option for nivolumab-monotherapy–appropriate patients with unresectable or metastatic melanoma.6-8
Opdualag
Opdualag is a dual immunotherapy option combining an anti–PD-1, nivolumab, with the first-in-class anti–LAG-3, relatlimab, in a fixed-dose formulation.7,8 PD-1 and LAG-3 are two distinct inhibitory immune checkpoints.7 Combined PD-1 and LAG-3 inhibition results in increased T-cell activation compared to the activity of either antibody alone. This initiates an improved anti-tumor immune response.8
Opdualag is indicated for the treatment of adult and pediatric patients 12 years of age or older with unresectable or metastatic melanoma.8 The approval is based on RELATIVITY-047, a phase 3, randomized, double-blind, global study of Opdualag versus nivolumab monotherapy.7 Patients were stratified by AJCC v8 M stage, BRAF, PD-L1, and LAG-3 status.7 Key exclusion criteria include patients with active or untreated brain or leptomeningeal metastases, uveal melanoma, active autoimmune disease, or medical conditions requiring systemic treatment with moderate- or high-dose corticosteroids or immunosuppressive medications.8
RELATIVITY-047 enrolled 714 patients who were randomized 1:1 to receive Opdualag (480 mg nivolumab/160 mg relatlimab as a fixed-dose combination[FDC]) every 4 weeks (n=355) or nivolumab 480 mg every 4 weeks (n=359).8 The primary endpoint was progression-free survival(PFS), and secondary endpoints were OS and overall response rate(ORR). PFS was determined by BICR using RECIST v1.1. Baseline characteristics were balanced across both treatment arms.7
Study design8
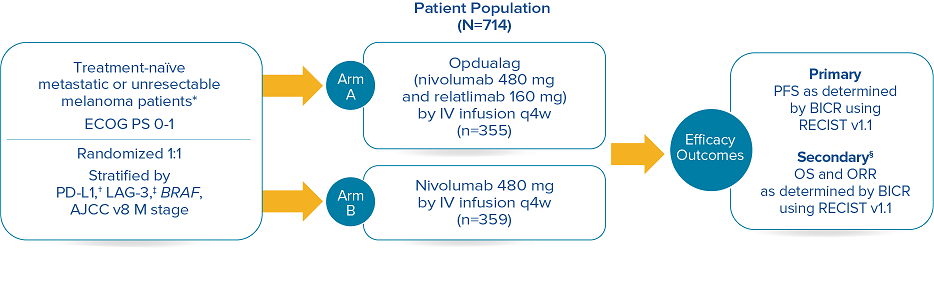
Median duration of treatment for Opdualag at the 19.3-month median follow-up was 8.3 months.7,9 Treat until disease progression or unacceptable toxicity.8
*Patients were allowed to have received prior adjuvant and neoadjuvant melanoma therapy. Anti–PD-1, anti–CTLA-4, or BRAF-MEK therapy was allowed as long as there was at least 6 months between the last dose of therapy and date of recurrence; interferon therapy was allowed as long as the last dose was at least 6 weeks prior to randomization.8† PD-L1 expression (≥1% vs <1%) using PD-L1 IHC 28-8 pharmDx test.8‡ LAG-3 expression (≥1% vs <1%) using a clinical trial assay.8§ The final analysis of OS was not statistically significant.8
Opdualag is associated with the following Warnings and Precautions: severe and fatal immune-mediated adverse reactions (IMARs) including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis with renal dysfunction, dermatologic adverse reactions, myocarditis, and other immune-mediated adverse reactions; infusion-related reactions; complications of allogeneic hematopoietic stem cell transplantation (HSCT); and embryo-fetal toxicity.
Opdualag demonstrated superior PFS compared to nivolumab at the primary analysis(median of 13.2 months) with curve separation as early as 3 months and sustained over time.7,8 Median PFS (mPFS)was 10.1 months with Opdualag versus 4.6 months with nivolumab (HR=0.75; 95% CI: 0.62–0.92; P=0.0055).8 Similarly, patients who received Opdualag had longer PFS regardless of key prognostic indicators, such as the AJCC metastasis stage of the tumor, LDH level, and tumor burden.7
At the follow-up analysis (median of 19.3 months), mPFS was 10.22 months with Opdualag and 4.63 months with nivolumab (HR=0.78; 95% CI: 0.64-0.94).10 OS and ORR were also evaluated.8 The final analysis for the secondary endpoint of OS was not statistically significant (threshold for significance was P<0.04302), and median OS (mOS)was not reached with Opdualag compared with nivolumab, which resulted in a mOS of 34.1 months (HR=0.80; 95% CI: 0.64–1.01; P=0.0593). Additionally, the ORR was higher with Opdualag (43%) versus nivolumab (33%), with the median DOR not yet reached for both treatment arms.8,10 ORR was not formally tested based on the testing hierarchy.8
Progression-free survival at the 19.3-month median follow-up10*†‡
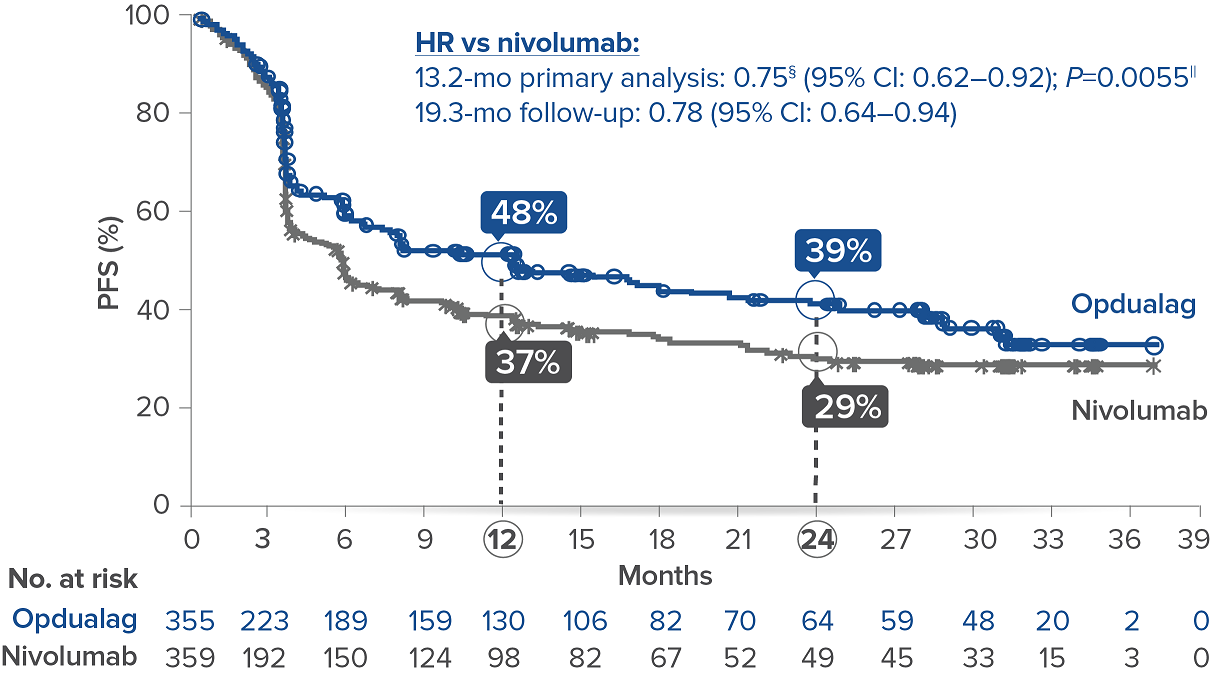
Symbols represent censored observations.
*Assessed by BICR.8† Final PFS analysis.8‡ Kaplan-Meier estimate.8§ Based on stratified Cox proportional hazard model.8II Based on stratified log-rank test.8
Overall survival10*
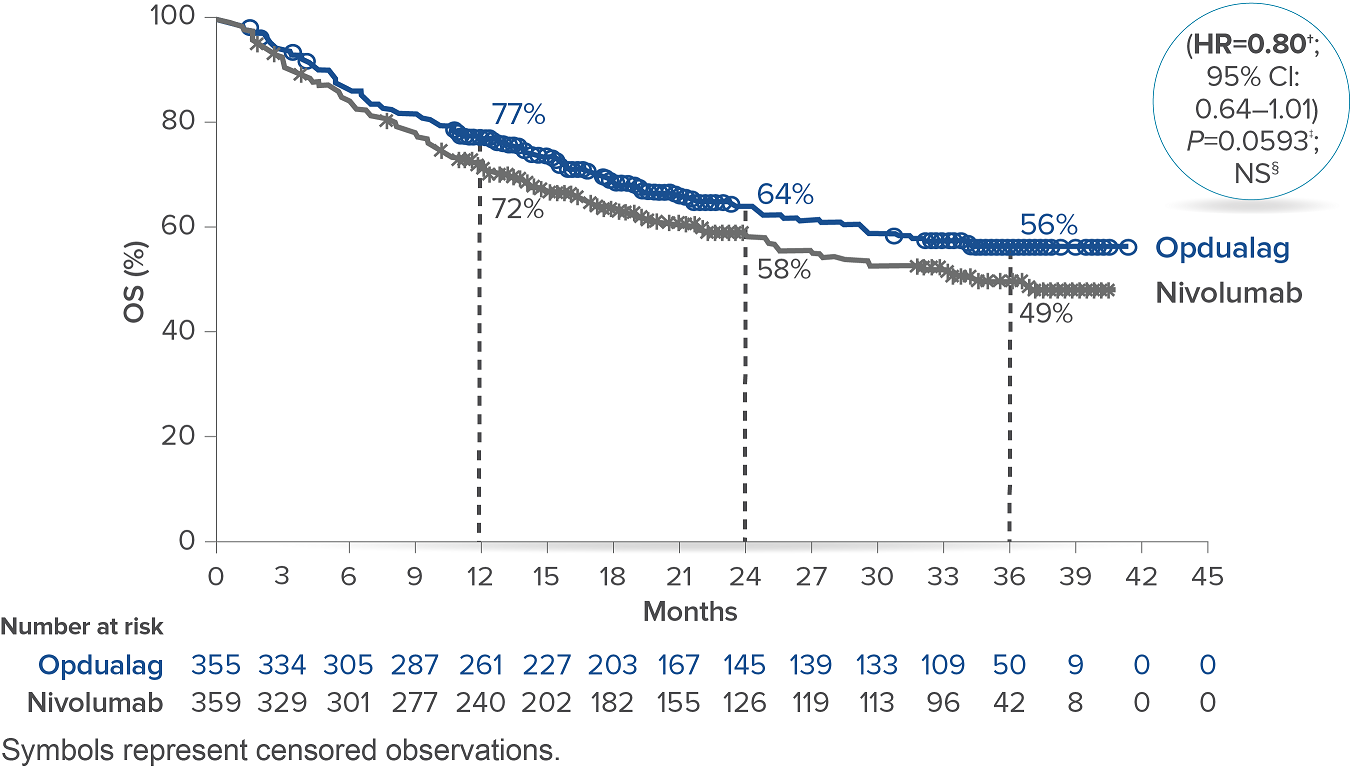
*At the time of the final OS analysis, which was event-driven and occurred after the final PFS analysis.8† Based on stratified Cox proportional hazard model.8‡ Based on stratified log-rank test.8§ Not significant at alpha level 0.04302.8
In RELATIVITY-047, Opdualag had no additional safety events and similar most common Grade 3/4 AEs versus nivolumab monotherapy.7,8 Adverse reactions occurring in ≥15% of patients receiving Opdualag were musculoskeletal pain (45%), fatigue (39%), rash (28%), pruritus (25%), diarrhea (24%), nausea (17%), headache (18%), hypothyroidism (17%), decreased appetite (15%), and cough (15%).8
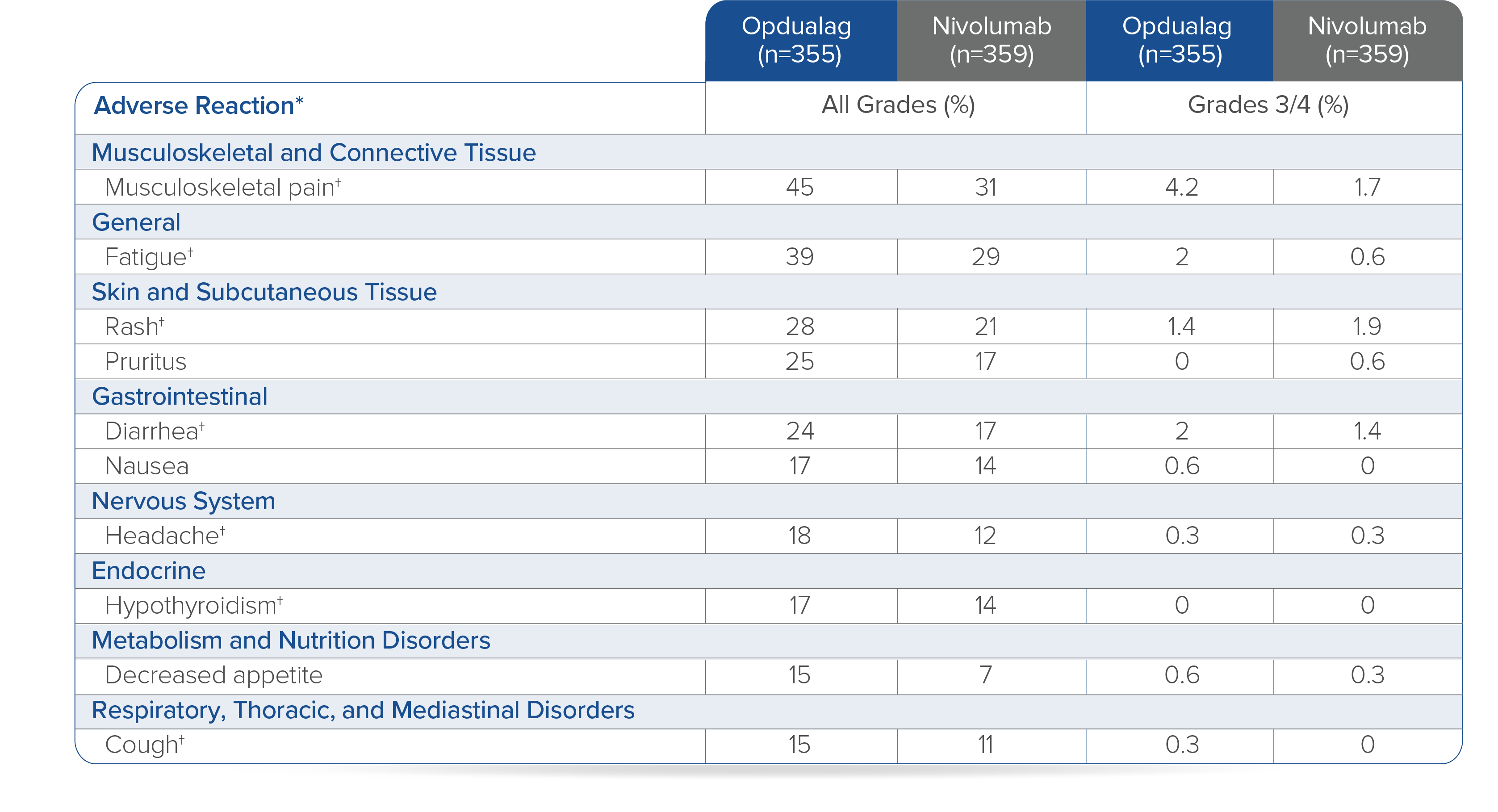
Toxicity was graded per NCI CTCAE v5.
*Clinically relevant adverse reactions in <15% of patients who received Opdualag included vitiligo, adrenal insufficiency, myocarditis, and hepatitis.8† Includes multiple terms.8
Opdualag is a FDC administered as a 30-minute intravenous infusion every 4 weeks.8 A FDC is the co-formulation of 2 active ingredients in a single vial administered as a single infusion, which may help reduce preparation and infusion times and could help minimize potential risk of administration errors.7,8,11 Opdualag can cause severe infusion-related reactions. Discontinue Opdualag in patients with severe or life-threatening infusion-related reactions. Interrupt or slow the rate of infusion in patients with mild to moderate infusion-related reactions. In patients who received Opdualag as a 60-minute intravenous infusion, infusion-related reactions occurred in 7% (23/355) of patients.8
Summary/conclusions
Dual immunotherapy has changed the metastatic melanoma treatment landscape.2 Currently there are 2 dual immunotherapy options available for 1L treatment of adult patients with unresectable or metastatic melanoma.3,8 As the newest dual immunotherapy, Opdualag more than doubled mPFS with a similar safety profile compared with nivolumab.8 Opdualag can be used for the treatment of all nivolumab monotherapy-appropriate patients, providing the opportunity for more patients with unresectable or metastatic melanoma to receive a dual immunotherapy.8 From my clinical experience, “it is great to have another treatment option for patients with metastatic melanoma.”
Indication for Opdualag
Opdualag is indicated for the treatment of adult and pediatric patients 12 years of age or older with unresectable or metastatic melanoma.
Important Safety Information for Opdualag
Severe and Fatal Immune-Mediated Adverse Reactions
Immune-mediated adverse reactions (IMARs) listed herein may not include all possible severe and fatal immune-mediated adverse reactions.
IMARs which may be severe or fatal, can occur in any organ system or tissue. IMARs can occur at any time after starting treatment with a LAG-3 and PD-1/PD-L1 blocking antibodies. While IMARs usually manifest during treatment, they can also occur after discontinuation of Opdualag. Early identification and management of IMARs are essential to ensure safe use. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying IMARs. Evaluate clinical chemistries including liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected IMARs, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue Opdualag depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). In general, if Opdualag requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose IMARs are not controlled with corticosteroid therapy. Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
Opdualag can cause immune-mediated pneumonitis, which may be fatal. In patients treated with other PD-1/PD-L1 blocking antibodies, the incidence of pneumonitis is higher in patients who have received prior thoracic radiation. Immune-mediated pneumonitis occurred in 3.7% (13/355) of patients receiving Opdualag, including Grade 3 (0.6%), and Grade 2 (2.3%) adverse reactions. Pneumonitis led to permanent discontinuation of Opdualag in 0.8% and withholding of Opdualag in 1.4% of patients.
Immune-Mediated Colitis
Opdualag can cause immune-mediated colitis, defined as requiring use of corticosteroids and no clear alternate etiology. A common symptom included in the definition of colitis was diarrhea. Cytomegalovirus infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
Immune-mediated diarrhea or colitis occurred in 7% (24/355) of patients receiving Opdualag, including Grade 3 (1.1%) and Grade 2 (4.5%) adverse reactions. Colitis led to permanent discontinuation of Opdualag in 2% and withholding of Opdualag in 2.8% of patients.
Immune-Mediated Hepatitis
Opdualag can cause immune-mediated hepatitis, defined as requiring the use of corticosteroids and no clear alternate etiology.
Immune-mediated hepatitis occurred in 6% (20/355) of patients receiving Opdualag, including Grade 4 (0.6%), Grade 3 (3.4%), and Grade 2 (1.4%) adverse reactions. Hepatitis led to permanent discontinuation of Opdualag in 1.7% and withholding of Opdualag in 2.3% of patients.
Immune-Mediated Endocrinopathies
Opdualag can cause primary or secondary adrenal insufficiency, hypophysitis, thyroid disorders, and Type 1 diabetes mellitus, which can be present with diabetic ketoacidosis. Withhold or permanently discontinue Opdualag depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information).
For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. In patients receiving Opdualag, adrenal insufficiency occurred in 4.2% (15/355) of patients receiving Opdualag, including Grade 3 (1.4%) and Grade 2 (2.5%) adverse reactions. Adrenal insufficiency led to permanent discontinuation of Opdualag in 1.1% and withholding of Opdualag in 0.8% of patients.
Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism; initiate hormone replacement as clinically indicated. Hypophysitis occurred in 2.5% (9/355) of patients receiving Opdualag, including Grade 3 (0.3%) and Grade 2 (1.4%) adverse reactions. Hypophysitis led to permanent discontinuation of Opdualag in 0.3% and withholding of Opdualag in 0.6% of patients.
Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism; initiate hormone replacement or medical management as clinically indicated. Thyroiditis occurred in 2.8% (10/355) of patients receiving Opdualag, including Grade 2 (1.1%) adverse reactions. Thyroiditis did not lead to permanent discontinuation of Opdualag. Thyroiditis led to withholding of Opdualag in 0.3% of patients. Hyperthyroidism occurred in 6% (22/355) of patients receiving Opdualag, including Grade 2 (1.4%) adverse reactions. Hyperthyroidism did not lead to permanent discontinuation of Opdualag. Hyperthyroidism led to withholding of Opdualag in 0.3% of patients. Hypothyroidism occurred in 17% (59/355) of patients receiving Opdualag, including Grade 2 (11%) adverse reactions. Hypothyroidism led to the permanent discontinuation of Opdualag in 0.3% and withholding of Opdualag in 2.5% of patients.
Monitor patients for hyperglycemia or other signs and symptoms of diabetes; initiate treatment with insulin as clinically indicated. Diabetes occurred in 0.3% (1/355) of patients receiving Opdualag, a Grade 3 (0.3%) adverse reaction, and no cases of diabetic ketoacidosis. Diabetes did not lead to the permanent discontinuation or withholding of Opdualag in any patient.
Immune-Mediated Nephritis with Renal Dysfunction
Opdualag can cause immune-mediated nephritis, which is defined as requiring use of steroids and no clear etiology. In patients receiving Opdualag, immune-mediated nephritis and renal dysfunction occurred in 2% (7/355) of patients, including Grade 3 (1.1%) and Grade 2 (0.8%) adverse reactions. Immune-mediated nephritis and renal dysfunction led to permanent discontinuation of Opdualag in 0.8% and withholding of Opdualag in 0.6% of patients.
Withhold or permanently discontinue Opdualag depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information).
Immune-Mediated Dermatologic Adverse Reactions
Opdualag can cause immune-mediated rash or dermatitis, defined as requiring use of steroids and no clear alternate etiology. Exfoliative dermatitis, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and Drug Rash with eosinophilia and systemic symptoms has occurred with PD-1/L-1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes.
Withhold or permanently discontinue Opdualag depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information).
Immune-mediated rash occurred in 9% (33/355) of patients, including Grade 3 (0.6%) and Grade 2 (3.4%) adverse reactions. Immune-mediated rash did not lead to permanent discontinuation of Opdualag. Immune-mediated rash led to withholding of Opdualag in 1.4% of patients.
Immune-Mediated Myocarditis
Opdualag can cause immune-mediated myocarditis, which is defined as requiring use of steroids and no clear alternate etiology. The diagnosis of immune-mediated myocarditis requires a high index of suspicion. Patients with cardiac or cardio-pulmonary symptoms should be assessed for potential myocarditis. If myocarditis is suspected, withhold dose, promptly initiate high dose steroids (prednisone or methylprednisolone 1 to 2 mg/kg/day) and promptly arrange cardiology consultation with diagnostic workup. If clinically confirmed, permanently discontinue Opdualag for Grade 2-4 myocarditis.
Myocarditis occurred in 1.7% (6/355) of patients receiving Opdualag, including Grade 3 (0.6%), and Grade 2 (1.1%) adverse reactions. Myocarditis led to permanent discontinuation of Opdualag in 1.7% of patients.
Other Immune-Mediated Adverse Reactions
The following clinically significant IMARs occurred at an incidence of <1% (unless otherwise noted) in patients who received Opdualag or were reported with the use of other PD-1/PD-L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions: Cardiac/Vascular: pericarditis, vasculitis; Nervous System: meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy; Ocular: uveitis, iritis, and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur. If uveitis occurs in combination with other IMARs, consider a Vogt-Koyanagi-Harada–like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss; Gastrointestinal: pancreatitis including increases in serum amylase and lipase levels, gastritis, duodenitis; Musculoskeletal and Connective Tissue: myositis/polymyositis, rhabdomyolysis (and associated sequelae including renal failure), arthritis, polymyalgia rheumatica; Endocrine: hypoparathyroidism; Other (Hematologic/Immune): hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection.
Infusion-Related Reactions
Opdualag can cause severe infusion-related reactions. Discontinue Opdualag in patients with severe or life-threatening infusion-related reactions. Interrupt or slow the rate of infusion in patients with mild to moderate infusion-related reactions. In patients who received Opdualag as a 60-minute intravenous infusion, infusion-related reactions occurred in 7% (23/355) of patients.
Complications of Allogeneic Hematopoietic Stem Cell Transplantation (HSCT)
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/PD-L1 receptor blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/PD-L1 blockade and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/PD-L1 receptor blocking antibody prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on its mechanism of action and data from animal studies, Opdualag can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Opdualag for at least 5 months after the last dose of Opdualag.
Lactation
There are no data on the presence of Opdualag in human milk, the effects on the breastfed child, or the effect on milk production. Because nivolumab and relatlimab may be excreted in human milk and because of the potential for serious adverse reactions in a breastfed child, advise patients not to breastfeed during treatment with Opdualag and for at least 5 months after the last dose.
Serious Adverse Reactions
In Relativity-047, fatal adverse reaction occurred in 3 (0.8%) patients who were treated with Opdualag; these included hemophagocytic lymphohistiocytosis, acute edema of the lung, and pneumonitis. Serious adverse reactions occurred in 36% of patients treated with Opdualag. The most frequent serious adverse reactions reported in ≥1% of patients treated with Opdualag were adrenal insufficiency (1.4%), anemia (1.4%), colitis (1.4%), pneumonia (1.4%), acute myocardial infarction (1.1%), back pain (1.1%), diarrhea (1.1%), myocarditis (1.1%), and pneumonitis (1.1%).
Common Adverse Reactions and Laboratory Abnormalities
The most common adverse reactions reported in ≥20% of the patients treated with Opdualag were musculoskeletal pain (45%), fatigue (39%), rash (28%), pruritus (25%), and diarrhea (24%).
The most common laboratory abnormalities that occurred in ≥20% of patients treated with Opdualag were decreased hemoglobin (37%), decreased lymphocytes (32%), increased AST (30%), increased ALT (26%), and decreased sodium (24%).
Please see US Full Prescribing Information for Opdualag.
Indication for OPDIVO® (nivolumab) + YERVOY® (ipilimumab)
OPDIVO, in combination with YERVOY, is indicated for the treatment of adult patients with unresectable or metastatic melanoma.
Important Safety Information
Severe and Fatal Immune-Mediated Adverse Reactions
Immune-mediated adverse reactions listed herein may not include all possible severe and fatal immune-mediated adverse reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. While immune-mediated adverse reactions usually manifest during treatment, they can also occur after discontinuation of OPDIVO or YERVOY. Early identification and management are essential to ensure safe use of OPDIVO and YERVOY. Monitor for signs and symptoms that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine, adrenocorticotropic hormone (ACTH) level, and thyroid function at baseline and periodically during treatment with OPDIVO and before each dose of YERVOY. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue OPDIVO and YERVOY depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). In general, if OPDIVO or YERVOY interruption or discontinuation is required, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy. Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
OPDIVO and YERVOY can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation. In patients receiving OPDIVO monotherapy, immune-mediated pneumonitis occurred in 3.1% (61/1994) of patients, including Grade 4 (<0.1%), Grade 3 (0.9%), and Grade 2 (2.1%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, immune-mediated pneumonitis occurred in 7% (31/456) of patients, including Grade 4 (0.2%), Grade 3 (2.0%), and Grade 2 (4.4%).
Immune-Mediated Colitis
OPDIVO and YERVOY can cause immune-mediated colitis, which may be fatal. A common symptom included in the definition of colitis was diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies. In patients receiving OPDIVO monotherapy, immune-mediated colitis occurred in 2.9% (58/1994) of patients, including Grade 3 (1.7%) and Grade 2 (1%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, immune-mediated colitis occurred in 25% (115/456) of patients, including Grade 4 (0.4%), Grade 3 (14%) and Grade 2 (8%).
Immune-Mediated Hepatitis and Hepatotoxicity
OPDIVO and YERVOY can cause immune-mediated hepatitis. In patients receiving OPDIVO monotherapy, immune-mediated hepatitis occurred in 1.8% (35/1994) of patients, including Grade 4 (0.2%), Grade 3 (1.3%), and Grade 2 (0.4%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, immune-mediated hepatitis occurred in 15% (70/456) of patients, including Grade 4 (2.4%), Grade 3 (11%), and Grade 2 (1.8%).
Immune-Mediated Endocrinopathies
OPDIVO and YERVOY can cause primary or secondary adrenal insufficiency, immune-mediated hypophysitis, immune-mediated thyroid disorders, and Type 1 diabetes mellitus, which can present with diabetic ketoacidosis. Withhold OPDIVO and YERVOY depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism; initiate hormone replacement as clinically indicated. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism; initiate hormone replacement or medical management as clinically indicated. Monitor patients for hyperglycemia or other signs and symptoms of diabetes; initiate treatment with insulin as clinically indicated.
In patients receiving OPDIVO monotherapy, adrenal insufficiency occurred in 1% (20/1994), including Grade 3 (0.4%) and Grade 2 (0.6%).In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, adrenal insufficiency occurred in 8% (35/456), including Grade 4 (0.2%), Grade 3 (2.4%), and Grade 2 (4.2%).
In patients receiving OPDIVO monotherapy, hypophysitis occurred in 0.6% (12/1994) of patients, including Grade 3 (0.2%) and Grade 2 (0.3%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, hypophysitis occurred in 9% (42/456), including Grade 3 (2.4%) and Grade 2 (6%).
In patients receiving OPDIVO monotherapy, thyroiditis occurred in 0.6% (12/1994) of patients, including Grade 2 (0.2%).
In patients receiving OPDIVO monotherapy, hyperthyroidism occurred in 2.7% (54/1994) of patients, including Grade 3 (<0.1%) and Grade 2 (1.2%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, hyperthyroidism occurred in 9% (42/456) of patients, including Grade 3 (0.9%) and Grade 2 (4.2%).
In patients receiving OPDIVO monotherapy, hypothyroidism occurred in 8% (163/1994) of patients, including Grade 3 (0.2%) and Grade 2 (4.8%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, hypothyroidism occurred in 20% (91/456) of patients, including Grade 3 (0.4%) and Grade 2 (11%).
In patients receiving OPDIVO monotherapy, diabetes occurred in 0.9% (17/1994) of patients, including Grade 3 (0.4%) and Grade 2 (0.3%), and 2 cases of diabetic ketoacidosis.
Immune-Mediated Nephritis with Renal Dysfunction
OPDIVO and YERVOY can cause immune-mediated nephritis. In patients receiving OPDIVO monotherapy, immune-mediated nephritis and renal dysfunction occurred in 1.2% (23/1994) of patients, including Grade 4 (<0.1%), Grade 3 (0.5%), and Grade 2 (0.6%).
Immune-Mediated Dermatologic Adverse Reactions
OPDIVO can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS) has occurred with PD-1/PD-L1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate nonexfoliative rashes.
YERVOY can cause immune-mediated rash or dermatitis, including bullous and exfoliative dermatitis, SJS, TEN, and DRESS. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-bullous/exfoliative rashes.
Withhold or permanently discontinue OPDIVO and YERVOY depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information).
In patients receiving OPDIVO monotherapy, immune-mediated rash occurred in 9% (171/1994) of patients, including Grade 3 (1.1%) and Grade 2 (2.2%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, immune-mediated rash occurred in 28% (127/456) of patients, including Grade 3 (4.8%) and Grade 2 (10%).
Other Immune-Mediated Adverse Reactions
The following clinically significant immune-mediated adverse reactions occurred at an incidence of <1% (unless otherwise noted) in patients who received OPDIVO monotherapy or OPDIVO in combination with YERVOY or were reported with the use of other PD-1/PD-L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions: cardiac/vascular: myocarditis, pericarditis, vasculitis; nervous system: meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy; ocular: uveitis, iritis, and other ocular inflammatory toxicities can occur; gastrointestinal: pancreatitis to include increases in serum amylase and lipase levels, gastritis, duodenitis; musculoskeletal and connective tissue: myositis/polymyositis, rhabdomyolysis, and associated sequelae including renal failure, arthritis, polymyalgia rheumatica; endocrine: hypoparathyroidism; other (hematologic/immune): hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis (HLH), systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection.
In addition to the immune-mediated adverse reactions listed above, across clinical trials of YERVOY monotherapy or in combination with OPDIVO, the following clinically significant immune-mediated adverse reactions, some with fatal outcome, occurred in <1% of patients unless otherwise specified: nervous system: autoimmune neuropathy (2%), myasthenic syndrome/myasthenia gravis, motor dysfunction; cardiovascular: angiopathy, temporal arteritis; ocular: blepharitis, episcleritis, orbital myositis, scleritis; gastrointestinal: pancreatitis (1.3%); other (hematologic/immune): conjunctivitis, cytopenias (2.5%), eosinophilia (2.1%), erythema multiforme, hypersensitivity vasculitis, neurosensory hypoacusis, psoriasis.
Some ocular IMAR cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada–like syndrome, which has been observed in patients receiving OPDIVO and YERVOY, as this may require treatment with systemic corticosteroids to reduce the risk of permanent vision loss.
Infusion-Related Reactions
OPDIVO and YERVOY can cause severe infusion-related reactions. Discontinue OPDIVO and YERVOY in patients with severe (Grade 3) or life-threatening (Grade 4) infusion-related reactions. Interrupt or slow the rate of infusion in patients with mild (Grade 1) or moderate (Grade 2) infusion-related reactions. In patients receiving OPDIVO monotherapy as a 60-minute infusion, infusion-related reactions occurred in 6.4% (127/1994) of patients. In a separate trial in which patients received OPDIVO monotherapy as a 60-minute infusion or a 30-minute infusion, infusion-related reactions occurred in 2.2% (8/368) and 2.7% (10/369) of patients, respectively. Additionally, 0.5% (2/368) and 1.4% (5/369) of patients, respectively, experienced adverse reactions within 48 hours of infusion that led to dose delay, permanent discontinuation or withholding of OPDIVO. In melanoma patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, infusion-related reactions occurred in 2.5% (10/407) of patients.
Complications of Allogeneic Hematopoietic Stem Cell Transplantation
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with OPDIVO or YERVOY. Transplant-related complications include hyperacute graft-versus-host-disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between OPDIVO or YERVOY and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with OPDIVO and YERVOY prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal studies, OPDIVO and YERVOY can cause fetal harm when administered to a pregnant woman. The effects of YERVOY are likely to be greater during the second and third trimesters of pregnancy. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with OPDIVO and YERVOY and for at least 5 months after the last dose.
Increased Mortality in Patients with Multiple Myeloma when OPDIVO is Added to a Thalidomide Analogue and Dexamethasone
In randomized clinical trials in patients with multiple myeloma, the addition of OPDIVO to a thalidomide analogue plus dexamethasone resulted in increased mortality. Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials.
Lactation
There are no data on the presence of OPDIVO or YERVOY in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment and for 5 months after the last dose.
Serious Adverse Reactions
In Checkmate 067, serious adverse reactions (74% and 44%), adverse reactions leading to permanent discontinuation (47% and 18%) or to dosing delays (58% and 36%), and Grade 3 or 4 adverse reactions (72% and 51%) all occurred more frequently in the OPDIVO plus YERVOY arm (n=313) relative to the OPDIVO arm (n=313). The most frequent (≥10%) serious adverse reactions in the OPDIVO plus YERVOY arm and the OPDIVO arm, respectively, were diarrhea (13% and 2.2%), colitis (10% and 1.9%), and pyrexia (10% and 1.0%).
Common Adverse Reactions
In Checkmate 067, the most common (≥20%) adverse reactions in the OPDIVO plus YERVOY arm (n=313) were fatigue (62%), diarrhea (54%), rash (53%), nausea (44%), pyrexia (40%), pruritus (39%), musculoskeletal pain (32%), vomiting (31%), decreased appetite (29%), cough (27%), headache (26%), dyspnea (24%), upper respiratory tract infection (23%), arthralgia (21%), and increased transaminases (25%). In Checkmate 067, the most common (≥20%) adverse reactions in the OPDIVO arm (n=313) were fatigue (59%), rash (40%), musculoskeletal pain (42%), diarrhea (36%), nausea (30%), cough (28%), pruritus (27%), upper respiratory tract infection (22%), decreased appetite (22%), headache (22%), constipation (21%), arthralgia (21%), and vomiting (20%).
Please see US Full Prescribing Information for OPDIVO and YERVOY.
References
1. Michielin O, Atkins MB, Koon HB, Dummer R, Ascierto PA. Evolving impact of long-term survival results on metastatic melanoma treatment. J Immunother Cancer. 2020. doi:10.1136/jitc-2020-000948.
2. Curti BD, Faries MB. Recent advances in the treatment of melanoma. N Engl J Med. 2021;384(23):2229-2240.
3. OPDIVO [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
4. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23-34.
5. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535-1546.
6. Cancer Research Institute. FDA Approval Timeline of Active Immunotherapies. Updated June 27, 2022. Accessed July 11, 2022. https://www.cancerresearch.org/en-us/scientists/immuno-oncology-landscape/fda-approval-timeline-of-active-immunotherapies.
7. Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24-34.
8. Opdualag [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
9. PubD 00058298. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
10. Long GV, Hodi FS, Lipson EJ, et al. Relatlimab and nivolumab vs nivolumab in previously untreated metastatic or unresectable melanoma: overall survival and response rates from RELATIVITY-047 (CA224-047). Oral presentation at ASCO Plenary Series 2022. Presentation number 9505.
11. US Food and Drug Administration. CFR–Code of Federal Regulations Title 21. Updated March 29, 2022. Accessed July 1, 2022.https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=300.50.
© 2022 Bristol-Myers Squibb Company. OPDIVO®, YERVOY®, Opdualag™, and the related logos are trademarks of Bristol-Myers Squibb Company.
7356-US-2200441 8/22
Additional Definitions
AJCC=American Joint Committee on Cancer; BICR=blinded independent central review; CI=confidence interval;CTLA-4=cytotoxic T-lymphocyte antigen 4; DOR=duration of response; ECOG PS=Eastern Cooperative Oncology Group Performance Status; HR=hazard ratio;IHC=immunohistochemistry; IV=intravenous;LAG-3=lymphocyte-activation gene 3; LDH=lactate dehydrogenase; M stage=metastasis stage; mo=month; no=number; NS=not significant; PD-1=programmed death receptor-1; PD-L1=programmed death ligand 1; q4w=every 4 weeks; RECIST=Response Evaluation Criteria In Solid Tumors.




