SUMMARY: The FDA on October 15, 2021, approved TECENTRIQ® (Atezolizumab) for adjuvant treatment, following resection and Platinum-based chemotherapy, in patients with Stage II to IIIA Non-Small Cell Lung Cancer (NSCLC) whose tumors have PD-L1 expression on 1% or more of tumor cells, as determined by an FDA-approved test. Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2021, about 235,760 new cases of lung cancer will be diagnosed and 131,880 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of NSCLC, 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large Cell Carcinomas. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, Adenocarcinoma now is the most frequent histologic subtype of lung cancer.
Surgical resection is the primary treatment for approximately 30% of patients with NSCLC who present with early Stage (I–IIIA) disease. These patients are often treated with platinum-based adjuvant chemotherapy to decrease the risk of recurrence. Nonetheless, 45-75% of these patients develop recurrent disease. There is therefore an unmet need for this patient population.
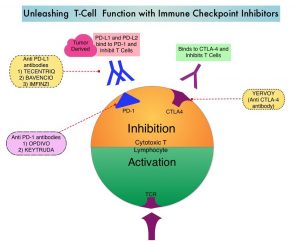
TECENTRIQ® is an anti PD-L1 monoclonal antibody, designed to directly bind to PD-L1 expressed on tumor cells and tumor-infiltrating immune cells, thereby blocking its interactions with PD-1 and B7.1 receptors expressed on activated T cells. PD-L1 inhibition may prevent T-cell deactivation and further enable the activation of T cells.
IMpower 010 is a global, multicentre, open-label, randomized Phase III study evaluating the efficacy and safety of TECENTRIQ® compared with Best Supportive Care (BSC), in patients with Stage IB-IIIA NSCLC, following surgical resection and up to 4 cycles of adjuvant Cisplatin-based chemotherapy. In this study, 1005 patients were randomized 1:1 to receive TECENTRIQ® 1200 mg IV every 3 weeks for 16 cycles, or BSC. Both study groups were well balanced and eligible patients had an ECOG PS of 0-1. The Primary endpoint was Disease Free Survival (DFS) in the PD-L1-positive Stage II-IIIA patients, all randomized Stage II-IIIA patients and Intent to Treat (ITT) Stage IB-IIIA populations. Key Secondary endpoints included Overall Survival (OS) in the overall study population and ITT Stage IB-IIIA NSCLC patients. At data cutoff on January 21, 2021, median follow up was 32.2 months in the ITT population.
Treatment with TECENTRIQ® following surgery and chemotherapy reduced the risk of disease recurrence or death (DFS-Disease Free Survival) by 34% (HR=0.66; P=0.0039), in patients with Stage II-IIIA NSCLC, whose tumor PD-L1 expression was 1% or more, compared with BSC. In this patient population, median DFS was Not Reached for TECENTRIQ®, compared with 35.3 months for BSC. This benefit was even more so among Stage II-IIIA NSCLC patients with PD-L1 expression 50% or more. Adjuvant TECENTRIQ® following surgery and chemotherapy in this patient group reduced the risk of disease recurrence or death (DFS) by 57% (HR=0.43). In the larger population of all randomized Stage II-IIIA study patients, TECENTRIQ® reduced the risk of disease recurrence or death by 21% (HR=0.79, P=0.02). In this patient population, TECENTRIQ® increased DFS by a median of seven months, compared with BSC (42.3 months versus 35.3 months). The significance boundary was not crossed for DFS in the ITT patient population. Overall Survival data were immature and not formally tested. Safety data for TECENTRIQ® were consistent with its known safety profile and no new safety signals were identified.
It was concluded that this study met its Primary endpoint, and is the first Phase III study to demonstrate that treatment with TECENTRIQ® following surgery and chemotherapy can significantly delay disease recurrence in patients with early stage lung cancer, with a more pronounced benefit noted, in patients with tumor PD-LI expression of 1% or more.
IMpower010: Primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC). Wakelee HA, Altorki NK, Zhou C, et al. J Clin Oncol. 2021;39:(suppl 15; abstr 8500). doi:10.1200/JCO.2021.39.15_suppl.8500