The FDA on March 3, 2023, approved VERZENIO® with endocrine therapy (Tamoxifen or an Aromatase Inhibitor) for the adjuvant treatment of adult patients with Hormone Receptor (HR)-positive, Human Epidermal Growth Factor Receptor 2 (HER2)-negative, node-positive, early breast cancer at high risk of recurrence. VERZENIO® is a product of Eli Lilly and Company.
Tag: Breast Cancer
TRODELVY® (Sacituzumab Govitecan-hziy)
The FDA on February 3, 2023, approved TRODELVY® for unresectable locally advanced or metastatic Hormone Receptor (HR)-positive, Human Epidermal Growth Factor Receptor 2 (HER2)-negative (IHC 0, IHC 1+ or IHC 2+/ISH-) breast cancer who have received endocrine-based therapy and at least two additional systemic therapies in the metastatic setting. TRODELVY® is a product of Gilead Sciences, Inc.
Targeting ESR1 Mutations in Estrogen-Positive Advanced Breast Cancer
Written By: Debra Patt, MD, PhD, MBA
In the golden age of oncology, many patients can now live with cancer as a chronic disease. Understanding how to optimally block cancer growth and how cancers develop mechanisms of resistance is critical to improving therapy.
For most patients with advanced breast cancer, estrogen blockade is the mainstay of early cancer treatments. Optimizing estrogen blockade in combination with other targets has dramatically improved progression-free and overall survival in patients with advanced breast cancer. Optimizing endocrine blockade in patients with ER+ advanced breast cancer is not only an effective therapy that improves outcomes, but also delays other systemic therapy, like chemotherapy, which have a toxicity profile that is typically more severe than endocrine therapy alone. By delaying chemotherapy with effective endocrine therapy, patients can enjoy longer disease-free intervals and maintain a high quality of life. While estrogen-positive breast cancer can be targeted by many estrogen-targeted therapies, resistance to aromatase inhibition through the development of ESR1 mutations is an important mechanism of resistance that contributes to cancer progression via the endocrine blockade.1
As we continue to make progress in cancer care, becoming familiar with new therapies is critical. This article will review elacestrant, approved by the Food and Drug Administration (FDA) in January 2023 for patients with estrogen receptor-positive (ER+) advanced breast cancer with ESR1 mutations after at least one line of endocrine therapy.
The superior response among patients with ESR1 mutations led to FDA approval among patients with ESR1 mutations who had received at least one line of endocrine therapy. Because ESR1 mutation status is central to FDA approval and the basis of many coverage determinations from payers, assessing ESR1 mutation status accurately is an important aspect of treatment. ESR1 mutations can develop in patients with ER+ advanced breast cancer and can change over time. In patients with treatment naïve early-stage breast cancer, de novo ESR1 mutations are relatively rare, but as patients are exposed to therapy, ESR1 mutations are acquired, making them a common mechanism of resistance in patients with metastatic disease.2 Because mutations develop over time with the evolutionary pressure of therapy, a patient’s ESR1 mutation status, when they are initially diagnosed with ER+ metastatic disease, can later change after exposure to aromatase inhibition. If analysis for ESR1 mutations is conducted early in a patient’s treatment and is found negative, resistance may emerge and only be demonstrated with subsequent molecular testing. There is evidence that blood-based serial testing may be a useful way to identify patients who are eligible for treatment.3 In January 2023, Guardant Health, through the Guardant 360 CDx, was approved by the FDA as a tool to test the blood for ESR1 mutations to assess for eligibility for elacestrant. By using sequential serologic testing, patients can have an assessment of molecular characteristics without undergoing additional biopsy. Because such a small number of patients have ESR1 mutations when they are treatment naïve, but it becomes much more likely through the course of a patient’s disease, repeat testing is the primary way to assess if ESR1 mutations have evolved over time, and can be conducted via plasma assessment.
Elacestrant works by binding estrogen receptor alpha and acting as a Selective Estrogen Receptor Down regulator (SERD), allowing degradation of the estrogen receptor. The FDA approved elacestrant in 2023 based on the reporting of the phase III EMERALD trial showing that patients with ER-positive and HER2 negative advanced breast cancer who had had one to two lines of endocrine therapy, pretreatment with a cyclin-dependent kinase 4/6 inhibitor, and not more than one line of chemotherapy, achieved a significant progression-free survival advantage when treated with elacestrant in comparison to other therapy.4 The population was further stratified as the whole population vs. just those with ESR1 mutations. In the entire population treated with elacestrant, PFS was prolonged (HR=0.70; 95% CI=0.55-0.88), and the results were more striking in those with ESR1 mutations (HR=0.55; 95% CI=0.39-0.77). In this group of pretreated patients with advanced breast cancer, ESR1 mutations were detected in 47.8% of patients. The progression-free survival of patients in the EMERALD trial was 3.8 months among patients receiving elacestrant in comparison to 1.9 months for other commonly prescribed endocrine therapies.
Elecestrant was well tolerated with treatment-related grade 3/4 adverse events in 7.2% of patients receiving elecestrant in comparison to 3.1% in patients receiving standard-of-care. Nausea was the most common side effect occurring to any extent in 35% of patients receiving elecestrant (though grade 3 was 2.5% and grade 4 was 0.9%) in comparison to 18.8% in patients who were receiving standard-of-care treatment. Other common side effects include abdominal pain, vomiting, diarrhea, constipation, elevation of liver function tests, cytopenias, hyponatremia, and fatigue. To mitigate side effects, it can help to take the medication with food, administer it at the same time each day, and use supportive anti-nausea and anti-diarrheal guidance upfront, in addition to dose reductions as appropriate.
In our modern era of cancer treatment, optimizing the use of incremental therapy can benefit patients. Making sure we consider ESR1 mutations in patients with ER+ advanced breast cancer, offer appropriate testing as patients are exposed to different treatments, and anticipate and mitigate side effects as appropriate will help us manage patients with ER+ advanced breast cancer optimally.
References
1) Brett, J.O., Spring, L.M., Bardia, A. et al. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res 23, 85 (2021). https://doi.org/10.1186/s13058-021-01462-3.
2) Kinslow CJ, Tang A, Chaudhary KR, Cheng SK. Prevalence of Estrogen Receptor Alpha (ESR1) Somatic Mutations in Breast Cancer. JNCI Cancer Spectr. 2022 Sep 1;6(5):pkac060. doi: 10.1093/jncics/pkac060. PMID: 35959983; PMCID: PMC9438742.
3) Sundaresan TK, Dubash TD, Zheng Z, Bardia A, Wittner BS, Aceto N, Silva EJ, Fox DB, Liebers M, Kapur R, Iafrate J, Toner M, Maheswaran S, Haber DA. Evaluation of endocrine resistance using ESR1 genotyping of circulating tumor cells and plasma DNA. Breast Cancer Res Treat. 2021 Jul;188(1):43-52. doi: 10.1007/s10549-021-06270-z. Epub 2021 Jun 8. PMID: 34101078; PMCID: PMC8667563.
4) Bidard FC, Kaklamani VG, Neven P, Streich G, Montero AJ, Forget F, Mouret-Reynier MA, Sohn JH, Taylor D, Harnden KK, Khong H, Kocsis J, Dalenc F, Dillon PM, Babu S, Waters S, Deleu I, García Sáenz JA, Bria E, Cazzaniga M, Lu J, Aftimos P, Cortés J, Liu S, Tonini G, Laurent D, Habboubi N, Conlan MG, Bardia A. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J Clin Oncol. 2022 Oct 1;40(28):3246-3256. doi: 10.1200/JCO.22.00338. Epub 2022 May 18. Erratum in: J Clin Oncol. 2023 Aug 10;41(23):3962. PMID: 35584336; PMCID: PMC9553388.
Omitting Radiotherapy after Breast-Conserving Surgery in Luminal A Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. It is estimated that approximately 300,590 new cases of breast cancer will be diagnosed in 2023 and about 43,700 individuals will die of the disease, largely due to metastatic recurrence. Breast cancer is the second leading cause of cancer death in women, in the U.S.
Patient undergoing breast conserving surgery, often receive adjuvant breast radiation therapy to reduce the risk of local recurrence. Radiation therapy however is inconvenient, expensive and is associated with acute and late toxicities. Previously published study by Kunkler IH, et al. (Lancet Oncol. 2015;16:266-273) concluded that radiotherapy could be avoided in a subset of elderly patients with low risk breast cancer following breast conserving surgery. However, conventional clinical pathological factors have limited ability to identify breast cancer patients with low risk disease, who could avoid radiation therapy. Even though biomarker assays such as 21-gene recurrence score and the Prediction Analysis of Microarray [PAM] 50 assay are being evaluated for their usefulness in identifying patients in whom radiotherapy might be omitted, follow-up in these trials is short. Molecular defined intrinsic subtypes of breast cancer may be of help in providing additional prognostic information.
Breast cancer is heterogeneous malignancy and using global gene expression analyses, 5 breast cancer intrinsic subtypes have been established. They include Luminal A, Luminal B, HER2-enriched, Basal-like, and Normal breast-like group. Luminal A breast cancer overexpresses estrogen pathway genes and is the least proliferative, and patients have the lowest risk of recurrence with the best prognosis. In a retrospective analysis of women over age 60 years, with Luminal A, Grade 1-2, T1N0 breast cancer, treated with breast conserving surgery and endocrine therapy alone, the local recurrence rate was low (JCO 2015; 33:2035). However, the utility of combining molecular subtype (Luminal A subtype) with clinical pathological factors, to guide radiotherapy decision-making, has not been prospectively evaluated.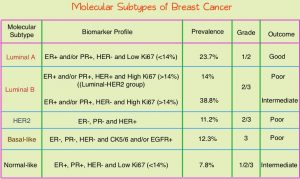
LUMINA is a prospective multicenter single-arm, cohort study, in which 500 women, 55 years and older, who had undergone breast conserving surgery for breast cancer, were enrolled. Eligible patients had invasive ductal T1N0, Grade 1-2, Luminal A breast cancer, had undergone breast conserving surgery, with excision margins of at least 1 mm and sentinel lymph node biopsy, omitted radiotherapy, and had received adjuvant endocrine therapy for at least 5 years. Luminal A subtype was defined as ER 1% or more, PR more than 20%, HER2 negative and Ki67 13.25% or less. Ki67 immunohistochemistry was performed centrally in one of three Canadian laboratories using International Ki67 Working Group methods. The median patient age was 67 years, 66% had Grade 1 tumors, 88% of patients were less than 75 years, and the median tumor size was 1.1 cm. Patients were excluded if they had a lobular carcinoma (including mixed ductal-lobular carcinoma), clinical or pathological evidence of direct extension to the chest wall or skin, multifocal or multicentric disease, Grade 3 histologic features, extensive intraductal component, or evidence of lymphovascular invasion. Patients were followed every six months for the first two years and then yearly. The Primary outcome was local recurrence defined as time from enrollment to any invasive or non-invasive cancer in the ipsilateral breast. Secondary endpoints included contralateral breast cancer, Disease Free Survival, and Overall Survival.
At a median follow up of 5 years, the local recurrence rate was 2.3% and the rate of contralateral breast cancer was 1.9%. The 5-year Disease Free Survival was 89.9% and 5-year Overall Survival rate was 97.2%.
The authors concluded that among women 55 years of age and over, with low grade Luminal A breast cancer, omission of radiation therapy following breast conserving surgery and treatment with endocrine therapy alone for 5 years or more, resulted in very low rates of local recurrence at 5 years. The researchers added that approximately 30,000-40,000 women per year in North America, predominantly in the US, could avoid the morbidity, expense, and inconvenience of radiotherapy.
Omitting Radiotherapy after Breast-Conserving Surgery in Luminal A Breast Cancer. Whelan TJ, Smith S, parpia S, et al. for the LUMINA Study Investigators. N Engl J Med 2023; 389:612-619.
Late Breaking Abstract – ASCO 2023: Toripalimab Plus Chemotherapy Improves Progression Free Survival in Metastatic Triple Negative Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. It is estimated that approximately 300,590 new cases of breast cancer will be diagnosed in 2023 and about 43,700 individuals will die of the disease, largely due to metastatic recurrence. Breast cancer is the second leading cause of cancer death in women, in the U.S.
Triple Negative Breast Cancer (TNBC) is a heterogeneous, molecularly diverse group of breast cancers and are ER (Estrogen Receptor), PR (Progesterone Receptor) and HER2 (Human Epidermal Growth Factor Receptor-2) negative. TNBC accounts for 15-20% of invasive breast cancers, with a higher incidence noted in young patients. It is usually aggressive, and tumors tend to be high grade and patients with TNBC are at a higher risk of both local and distant recurrence. Those with metastatic disease have one of the worst prognoses of all cancers with a median Overall Survival of 13 months. The majority of patients with TNBC who develop metastatic disease do so within the first 3 years after diagnosis, whereas those without recurrence during this period of time have survival rates similar to those with ER-positive breast cancers.
Previously published studies have shown that presence of tumor-infiltrating lymphocytes was associated with clinical benefit, when treated with chemotherapy and immunotherapy, in patients with TNBC, and improved clinical benefit was observed in patients with immune-enriched molecular subtypes of metastatic TNBC. Toripalimab, a checkpoint inhibitor, is a humanized IgG4K monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. It thereby reverses the PD-1 pathway-mediated inhibition of the immune response, and unleashes the tumor-specific effector T cells. Toripalimab provided significant clinical activity with a favorable safety profile in several solid tumors.
The purpose of this study is to compare the efficacy and safety of Toripalimab versus placebo, in combination with nab-Paclitaxel for metastatic or recurrent TNBC. The rationale for combining chemotherapy with immunotherapy is that cytotoxic chemotherapy releases tumor-specific antigens, and immune checkpoint inhibitors such as Toripalimab when given along with chemotherapy can enhance endogenous anticancer immunity.
TORCHLIGHT is a randomized, double-blind, placebo-controlled, multi-center, Phase III trial, in which the safety and efficacy of Toripalimab plus nab-Paclitaxel was compared with placebo plus nab-Paclitaxel in patients with Stage IV or recurrent/metastatic TNBC. In this study, 531 (N=531) eligible patients were randomly assigned 2:1 to receive Toripalimab 240mg IV on Day 1 every 3 weeks (N=353) or placebo (N=178), along with nab-Paclitaxel given at 125 mg/m2 on days 1 and 8 of each cycle. Treatment was continued until disease progression or intolerable toxicity. Patients could not have received more than one line of chemotherapy in the metastatic setting and had to be eligible for taxane monotherapy. Baseline characteristics were well balanced between the treatment groups and patients were stratified based on PD-L1 expression, Paclitaxel therapy history and line of prior therapy at enrollment. In the Toripalimab group, 200 patients had PD-L1 positive disease, whereas 100 patients in the placebo group had PD-L1-positive disease. The Primary endpoint was Progression Free Survival (PFS) assessed by a Blinded Independent Central Review (BICR), first in the PD-L1-positive population and then in the Intent-To-Treat (ITT) population. Secondary endpoints included Overall Survival (OS), Objective Response Rate (ORR), Duration of Response (DoR), Disease Control Rate and Safety.
At interim analysis, with the median follow up of 14 months, a statistically significant improvement in PFS was demonstrated with Toripalimab in the PD-L1 positive subgroup. The median PFS was 8.4 months versus 5.6 months respectively (HR=0.65; P=0.01). The PFS in the overall population showed a similar trend and was 8.4 months in the Toripalimab group and 6.9 months in the placebo group (HR=0.77; P=0.04). A descriptive Overall Survival analysis showed a trend towards improved OS with Toripalimab in the PD-L1 positive group (median OS 32.8 versus 19.5 months; HR=0.61; P=0.01). In the overall population, the median OS was 33.1 versus 23.5 months (HR=0.69, P=0.01). No new safety signals were identified.
The authors concluded that, for PD-L1 positive metastatic or recurrent Triple Negative Breast Cancer patients receiving first-line treatment, the addition of Toripalimab to nab-Paclitaxel resulted in a significant improvement in Progression Free Survival with an acceptable safety profile. Patients are being followed for the final PFS and OS analysis.
TORCHLIGHT: A randomized, double-blind, phase III trial of toripalimab versus placebo, in combination with nab-paclitaxel(nab-P) for patients with metastatic or recurrent triple-negative breast cancer (TNBC). Jiang Z, Ouyang Q, Sun T, et al. J Clin Oncol 41, 2023 (suppl 17; abstr LBA1013)
Ovarian Ablation or Suppression May Lower the Risk of Breast Cancer Recurrence
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. It is estimated that approximately 300,590 new cases of breast cancer will be diagnosed in 2023 and about 43,700 individuals will die of the disease, largely due to metastatic recurrence. Breast cancer is the second leading cause of cancer death in women, in the U.S. About 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors, and HR-positive/HER2-negative breast cancer is the most frequently diagnosed molecular subtype. About 90% of all breast cancers are detected at an early stage, and these patients are often cured with a combination of surgery, radiotherapy, chemotherapy, and hormone therapy.
It has been hypothesized that estrogen in breast cancer acts as a catalyst/promoter for cancer growth, by stimulating the division and proliferation of breast tissue and increasing the risk for cancer causing mutations. A recently published study (Nature 2023;618:1024–1032) suggests that estrogen might be involved in the genomic reshuffling that gave rise to cancer-gene activation in breast cancer, acting as an initiator as well.
The researchers in this study postulated that suppressing ovarian function of women with breast cancer may improve outcome by preventing estrogenic stimulation of any residual/microscopic cancer, particularly among pre-menopausal women with Estrogen Receptor (ER)-positive tumors. To further clarify this benefit, the researchers from the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) conducted a patient-level meta-analysis of 14,993 pre-menopausal women in 25 randomized trials, that compared ovarian ablation or suppression with no ovarian ablation or suppression. Primary analyses included only premenopausal women age less than 55 years, with ER-positive or unknown tumors, stratified into those who received no chemotherapy, or remained premenopausal following chemotherapy, and those whose menopausal status following chemotherapy was not ascertained.
The following observations were noted from this meta-analysis:
–Fewer breast cancer recurrences were seen overall with ovarian ablation/suppression than control (RR=0.82, P< 0.0001).
• Among women receiving no chemotherapy or remaining premenopausal after chemotherapy (N=7,213), similar benefits were seen and the reduction in recurrent breast cancer was significant with ovarian ablation/suppression than control. The breast cancer recurrence rate at 15 years was 39.3% in the control group versus 29.5% in the ovarian ablation or suppression group, with an absolute benefit of 9.8% and a Rate Ratio (RR) of 0.71 (P<0.0001).
• Breast cancer mortality and all-cause mortality in the ovarian ablation or suppression group at 15 years, were improved by 8.0% (20.9% versus 28.9%; RR 0.69, P<0.0001) and 7.2% (26.0% versus 33.1%; RR = 0.73, P< 0.0001), respectively, with no increase in deaths without recurrence (RR = 0•88, P=0.33).
• Among those women who were premenopausal before chemotherapy and whose menopausal status was uncertain after chemotherapy (N=7,786), the rate of recurrence at 15 years was 43.1% in those who received ovarian ablation/suppression and 44.4% in the control group (RR=0.91; P =0.03).
• Recurrence reductions were significantly larger among premenopausal women under 45 years, than among those 45-54 years, and did not differ significantly by tumor characteristics. Among premenopausal women under 45 years (N=4,437), the recurrence rate was 41.3% in the control group and 30.4% with ovarian ablation or suppression, representing a 15-year benefit of 10.9% and a Rate Ratio of 0.66 (P<0.00001). Among those women 45-54 years (N=2,776), the recurrence rate was 36.1% in the control group and 28.6% with ovarian ablation or suppression, suggesting a 15-year benefit of 7.5% and Rate Ratio of 0.82 (P=0.02).
• Among those taking Tamoxifen, the benefit with ovarian ablation or suppression was less, and was only 4.5% (RR = 0.80; P =0.002).
The authors concluded that ovarian ablation or suppression in pre-menopausal women less than 45 years with ER-positive breast cancer, substantially reduces the 15-year risk of recurrence and death from breast cancer, without increasing mortality from other causes.
Effects of ovarian ablation or suppression on breast cancer recurrence and survival: Patient-level meta-analysis of 14,993 pre-menopausal women in 25 randomized trials. Gray RG, Bradley R, Braybrooke J, et al. J Clin Oncol 41, 2023 (suppl 16; abstr 503)
Late Breaking Abstract – ASCO 2023: Chemotherapy De-escalation using PET Response-Adapted Strategy in Patients with HER2-positive Early Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. It is estimated that approximately 300,590 new cases of breast cancer will be diagnosed in 2023 and about 43,700 individuals will die of the disease, largely due to metastatic recurrence. Breast cancer is the second leading cause of cancer death in women, in the U.S.
The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. Adjuvant and neoadjuvant chemotherapy given along with anti-HER2 targeted therapy reduces the risk of disease recurrence and death, among patients with HER2-positive, early stage, as well as advanced metastatic breast cancer.
Trastuzumab is a humanized monoclonal antibody targeting HER2. It binds to the extracellular subdomain IV of the receptor and disrupts ligand independent HER2 downstream cell signaling pathways. Pertuzumab is a recombinant, humanized, monoclonal antibody that binds to the HER2 subdomain II and blocks ligand dependent HER2 heterodimerization with other HER receptors. Thus Trastuzumab along with Pertuzumab provide a more comprehensive blockade of HER2 driven signaling pathways. Dual HER2 blockade with Trastuzumab and Pertuzumab, given along with chemotherapy (with or without endocrine therapy), as first line treatment, in HER2 positive metastatic breast cancer patients, was shown to significantly improve Progression Free Survival (PFS) as well as Overall Survival (OS). The superior benefit with dual HER2 blockade has been attributed to differing mechanisms of action and synergistic interaction between HER2 targeted therapies.
Pathological Complete Response (pCR) after neoadjuvant therapy has strong prognostic significance in HER2+ breast cancer, and pCR rates in HER2+/HR- negative tumors exceed those in HER2+/HR+ tumors, and this in turn correlates with superior Event Free Survival. The FDA approved anti-HER2 dual blockade with Pertuzumab and Trastuzumab, given along with chemotherapy for the neoadjuvant treatment of patients with HER2-positive, locally advanced, inflammatory, or early stage breast cancer, based on the NeoSphere trial, and for metastatic disease based on positive survival results in the CLEOPATRA trial. The role of chemotherapy free anti-HER2 dual blockade however has remained unclear.
PHERGain is a multinational, multicenter, randomized, open-label, non-comparative, Phase II trial, designed to explore the feasibility of dual HER2 blockade with a chemotherapy de-escalation strategy, using a response-adapted design. This study design allowed the identification of treatment responders earlier in the study, and non-responders were switched to Standard-of-Care treatment. In this study, 356 patients with Stage I-IIIA, invasive, HER2-positive operable breast cancer, with tumor size 1.5 cm or more, and with at least one breast lesion evaluable by FDG-PET, were included. Patients were randomized 1:4 to receive either Docetaxel 75 mg/m2 IV, Carboplatin AUC 6 mg/mL IV, Trastuzumab 600 mg SC given as a fixed dose, and Pertuzumab 840 mg IV given as a loading dose, followed by 420 mg IV maintenance doses (TCHP) – Group A (N=71) or Trastuzumab and Pertuzumab alone (HP) – Group B (N=285). Patients were stratified by Hormone Receptor status and Hormone Receptor-positive patients allocated to Group B were additionally given Letrozole if postmenopausal (2.5 mg/day orally) or Tamoxifen if premenopausal (20 mg/day orally). Centrally reviewed FDG-PET scans were done before randomization and after two treatment cycles. Patients assigned to Group A completed six cycles of treatment (given every 3 weeks) regardless of FDG-PET results. Patients assigned to Group B initially received two cycles of Trastuzumab and Pertuzumab. FDG-PET responders (reduction in breast lesions of at least 40% from baseline) in Group B continued this treatment for six additional cycles. FDG-PET non-responders in this group were switched to six cycles of Docetaxel, Carboplatin, Trastuzumab, and Pertuzumab (TCHP). Surgery was performed 2-6 weeks after the last dose of study treatment. Adjuvant treatment was selected according to the neoadjuvant treatment administered, pathological response, hormone receptor status, and clinical stage at diagnosis. The co-Primary endpoints were the proportion of FDG-PET responders in Group B with a pathological Complete Response in the breast and axilla (ypT0/is ypN0), as determined by a local pathologist after surgery, following eight cycles of treatment, as well as 3-year invasive Disease-Free Survival (DFS) of patients in Group B. In an earlier analysis of this study, at a median follow-up was 5.7 months, 80% of the patients in Group B were FDG-PET responders, of whom 38% had a pathological Complete Response, achieving the first Primary endpoint (Perez-Garcia JM, Lancet Oncol 2021).
The researchers herein presented the results of the second Primary endpoint, 3-year invasive DFS, among patients included in Group B who underwent surgery based on an Intent-to-Treat (ITT) analysis. In Group A, 89% proceeded to surgery and 93.7% proceeded to have surgery in Group B. The 3-year invasive DFS among the 80% of patients in Group B who were FDG-PET responders was 95.4%, meeting the second Primary endpoint (P<0.001). Further, a subgroup analysis showed that of the patients in Group B who were FDG-PET responders and who also achieved a pathological Complete Response (38%), none received chemotherapy at any point in the 3 years they were in the study. These patients had a 3-year invasive DFS of 98.8%, and only one patient experienced invasive event (locoregional ipsilateral recurrence).
As expected, treatment-related Adverse Events and serious Adverse Events were significantly higher in patients assigned to Group A than to Group B, and Group B patients with pathological Complete Response had the lowest incidence of Grade 3 or more Adverse Events.
The authors concluded that among patients with HER2-positive early operable breast cancer, a PET-based, pathological Complete Response-adapted strategy was associated with a substantial 3-year invasive Disease Free Survival. The authors added that this treatment approach identifies about a third of HER2-positive early breast cancer patients who may safely omit chemotherapy and avoid the risk of treatment-related toxicities.
3-year invasive disease-free survival (iDFS) of the strategy-based, randomized phase II PHERGain trial evaluating chemotherapy (CT) de-escalation in human epidermal growth factor receptor 2-positive (HER2[+]) early breast cancer (EBC). Cortes J, Pérez-García JM, Ruiz-Borrego M, J Clin Oncol 41, 2023 (suppl 17; abstr LBA506)
Fixed Dose versus Standard Dose Capecitabine in Metastatic Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. It is estimated that approximately 300,590 new cases of breast cancer will be diagnosed in 2023 and about 43,700 individuals will die of the disease, largely due to metastatic recurrence. Breast cancer is the second leading cause of cancer death in women, in the U.S. Approximately 70% of breast tumors in patients with metastatic disease are Estrogen Receptor (ER) and/or Progesterone Receptor (PR) positive and HER2-negative. These patients are often treated with single agent endocrine therapy, endocrine therapy in combination with CDK4/6 inhibitor, or single agent chemotherapy. Resistance to hormonal therapy occurs in a majority of the patients.
Capecitabine (XELODA®) is one of the most frequently prescribed chemotherapeutic agents, for the treatment of breast cancer, and patients with metastatic breast cancer often receive Capecitabine following progression on anthracycline and taxane-based therapy. Capecitabine is preferred as it is not associated with alopecia or neuropathy, and can be administered orally. The FDA approved dosing schedule for Capecitabine is 1250 mg/m2 orally twice daily 14 days on, 7 days off. This dosing and schedule however is associated with poor tolerance and high discontinuation rates. Mathematical models suggest that a fixed, dose-dense schedule may be optimal for Capecitabine efficacy.
The X-7/7 is a randomized Phase II study in which the efficacy and tolerability of fixed-dose Capecitabine was compared with standard-dose Capecitabine, in patients with metastatic breast cancer. This study included 153 patients who were randomly assigned in a 1:1 ratio to receive either fixed-dose Capecitabine at 1500 mg orally twice daily, 7 days on followed by 7 days off (N=80) or the FDA approved standard-dose Capecitabine at 1250 mg/m2 twice daily, 14 days on followed by 7 days off (N=73). Female patients with metastatic breast cancer, regardless of the number of prior lines of endocrine therapy or chemotherapy they had received, were included. HER-2 positive patients were allowed with concurrent Trastuzumab. Majority of patients included in this study had Hormonal Receptor (HR)-positive, HER2-negative disease, 11% were HER-2 positive, 11% were triple negative, and 65% of patients were chemotherapy-naïve. Patients were stratified by line of chemotherapy (first line or subsequent), measurable disease, and ER status. The Primary endpoint was 3-month Progression Free Survival (PFS). Additional endpoints included PFS and Overall Survival (OS).
It was noted that the fixed dosing schedule of Capecitabine was associated with less toxicity and similar survival when compared with the standard dosing schedule. The 3-month PFS was similar at 76% in both the fixed-dose group and standard-dose group (HR=1.01; P=0.99). Landmark analysis of PFS at 12, 24 and 36 months for fixed-dose versus standard-dose Capecitabine was 39% versus 50% at 12 months (P=0.23), 25% versus 23% at 24 months (P=0.77), and 11% versus 0% at 36 months (P=0.24), respectively. The restricted mean PFS at 36 months was 13.9 months in the fixed-dose group versus 14.6 months in the standard-dose group (HR=1.31; P=0.24). The restricted mean OS at 36 months was 21.2 months versus 19.6 months, respectively (HR=0.80; P=0.27)
Patients receiving fixed-dose Capecitabine were less likely to experience Grade 2-4 toxicities than those receiving standard-dose Capecitabine (25% versus 49.3%, P=0.0018). Treatment discontinuation due to toxicities was significantly lower with fixed-dose Capecitabine compared with standard-dose Capecitabine (7.5% versus 28.8%, respectively, P<0.0006).
It was concluded that fixed dose Capecitabine 1500 mg orally twice daily 7 days on followed by 7 days off, has less toxicity and may improve tolerability without compromising efficacy, compared to the standard BSA-based dosing 14 days on followed by 7 days off. For patients receiving Capecitabine in an adjuvant setting with a curative intent (e.g. CREATE-X trial), standard BSA-based dosing and schedule is appropriate.
Randomized trial of fixed dose capecitabine compared to standard dose capecitabine in metastatic breast cancer: the X-7/7 tria. Khan QJ, Bohnenkamp C, Monson T, et al. DOI:10.1200/JCO.2023.41.16_suppl.1007 Journal of Clinical Oncology 41, no. 16_suppl (June 01, 2023) 1007-1007.
Clinical Pearls on Abemaciclib
Written by: Debra Patt, MD, PhD, MBA
In our lifetime, the CDK 4/6 inhibitors have improved the quality of life and progression-free survival for patients with estrogen receptor (ER)-positive/human epidermal growth factor 2- (HER2)-negative breast cancer more than any other drug. Giving patients the opportunity for treatment allows them to realize the dream of modern cancer therapy. Over time, these drugs continue to show great promise in the metastatic setting and in high-risk adjuvant breast cancer patients. Understanding their optimal use and managing their toxicity will get us closer to supporting our patients to live well without cancer. This article will address abemaciclib in metastatic breast cancer and also its use in early-stage breast cancer, including the update of FDA guidance and also data including 4-year follow up.
Abemaciclib in Metastatic Breast Cancer
The first CDK4/6 inhibitor palbociclib, was approved by the FDA in 2016, followed by ribociclib and abemaciclib which were approved the following year. These drugs as a class have made a palpable difference in the lives of breast cancer patients. They have not only improved progression-free and overall survival but have also allowed patients with advanced cancer to live with the disease without the burden of highly toxic intravenous chemotherapy. In that way, many patients control their cancer just like hypertension or other chronic illnesses, with pills that have minimal impact on their quality of life.
The three CDK4/6 inhibitors are often discussed comparatively, but we do not yet have direct comparative data, limiting decisions on therapy to our understanding of each of them individually and their efficacy and toxicity profiles.
Some differences of importance across the drugs in the metastatic setting are efficacy and toxicity. See Table 1 for the designs of the metastatic trials and their efficacy in comparison to the control arms. In addition, there are important differences in adverse effect profiles, seen in Table 2. It is notable that in the frontline trials, many patients were managed with dose reduction. This is an important point that will be touched upon again and again, that there is no compelling evidence that efficacy is sacrificed when dose reduction is managed to abate toxicity. More specifically, given the absence of data on dose response curves and the high rates of discontinuation due to toxicity, practitioners should be eager to manage symptoms with supportive care medications and dose reduction. Specifically, when we initiate patients on treatment with abemaciclib, they should be followed closely—initially, weekly or every other week—and dose should be reduced rapidly as indicated to manage symptoms. Similarly empowering patients with education and administering anti-diarrheal therapy to manage toxicity with initial prescribing can go a long way to assist in symptom control. Taking these actions up front could prevent early discontinuation of effective therapy.
Table 1: Summary data of efficacy of frontline CDK4/6 inhibitors in postmenopausal ER-positive breast cancer patients.
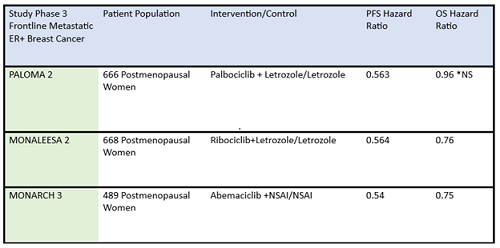
ER+, estrogen receptor positive; NS, not significant; NSAI, nonsteroidal aromatase inhibitor; OS, overall survival; PFS, progression-free survival
*Paloma 2 hazard ratio for OS was not statistically significant
Table 2: Summary of adverse events (AE) and serious adverse events (SAE) of frontline CDK4/6 inhibitors in post-menopausal ER-positive breast cancer patients
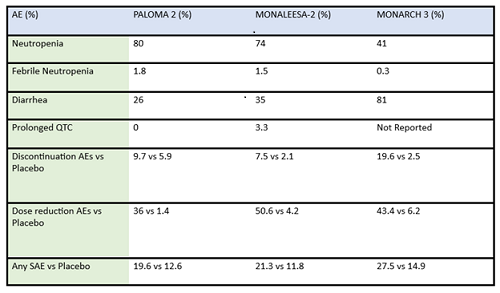
There are some key differences in how CDK4/6 inhibitors are used in the metastatic setting: activity in combination vs as a single agent, penetration of the blood brain barrier, and evidence for benefit from treatment after progressing on another drug in the same class. For example, abemaciclib is FDA approved as a single agent showing activity with doses at 200mg every 12 hours for patients with metastatic ER-positve/HER2-negative breast cancer1. Abemaciclib has activity in the central nervous system, and is included in the ASCO guidelines among the active agents in ER-positive/HER2-amplified breast cancer with brain metastasis2. Abemaciclib may be an effective therapy after treatment with palbociclib, as a recent cohort of 52 patients previously treated with palbociclib exhibited a clinically meaningful benefit from subsequent therapy with abemaciclib3.
Abemaciclib in Adjuvant High-Risk ER-Positive/HER2-Negative Breast Cancer
Observing the success in patients with metastatic breast cancer, we are seeking to understand if treatment is beneficial in earlier lines of therapy. The MONARCH E trial, evaluating the efficacy and safety of abemaciclib in combination with endocrine blockade in patients with node-positive high-risk ER-positive breast cancer, demonstrated an improvement in disease-free survival. This has been a clinically meaningful addition to our armamentarium of treatment, although careful consideration of management is important as early failure to manage adverse effects can lead to early discontinuation. According to the 4-year follow-up data from MONARCH E, the median invasive disease-free survival benefit previously reported of HR=0.664 (95% CI 0.578-0.762, nominal p<0.0001) was persistent and the absolute difference in invasive disease-free survival was 6.4% (85.8% in the endocrine therapy plus abemaciclib arm versus 79.4% in the endocrine only arm). Overall survival did not meet statistical significance, and the adverse effect profile reflected toxicities known to be associated with abemaciclib, including neutropenia, leukopenia, and diarrhea4. Adjuvant abemaciclib was approved by the FDA in 2021 and is currently approved in combination with endocrine therapy (tamoxifen or an aromatase inhibitor) for the adjuvant treatment of adult patients with HR-positive, HER2-negative, node-positive early breast cancer at high risk of recurrence. Of note, in March 2023, the FDA approval was expanded to remove Ki-67 >20% as a qualifying factor for approval. Patients defined as high risk included those having ≥4 pathologically involved axillary lymph nodes or 1-3 axillary lymph nodes and either tumor grade 3 or tumor size >5cm.
Abemaciclib causes GI toxicity in the form of cramping and diarrhea. Frequently, patients are afflicted with this toxicity, and if they are not optimally managed with anti-diarrheal agents and dose reductions, the patients will prematurely discontinue effective therapy. This is a particular problem in the adjuvant patients: they have often already completed systemic chemotherapy, and their therapeutic enthusiasm wanes as they have completed what they often (incorrectly) perceive as the more important part of therapy. Critical attention to symptom management, patient education, and dose reduction are important, as prescribing at the FDA approved dose will sometimes cause intolerable adverse effects, and early dose reduction will likely lead to reduction of adverse effects and improved compliance with the adjuvant treatment strategy. With all of the CDK4/6 inhibitors there is a large amount of inter-individual variability in exposure, yet in contrast to palbociclib and ribociclib, abemaciclib has three active metabolites that all have clinical activity5. As we don’t have a robust amount of clinical data on dose response to abemaciclib, there has been some hesitation among practitioners to implement strategies to manage toxicity early with dose reduction. Anecdotally, some strategies that have been effective in managing adverse effects include giving a smaller allocation of the drug and seeing the patient 1 and 2 weeks out in follow up, quickly reducing the dose, and sometimes even starting at a lower dose initially. In addition, partnering a new therapy regimen with patient education and loperamide to manage adverse effects can assist in helping patients avoid and manage severe toxicity.
The biggest challenge I have anecdotally observed in clinical practice in patients benefitting from adjuvant abemaciclib is that qualifying patients often don’t have it prescribed for them as part of their adjuvant therapy. Adjuvant abemaciclib was approved in 2021 by the FDA, and while adoption does take time, adoption in clinical practice has been variable.
Clinical Take Aways: When prescribing abemaciclib in patients with metastatic breast cancer, patient education, up-front management of diarrhea, and close follow-up for dose modification and symptom management needs are critical. When prescribing abemaciclib in patients with high-risk ER-positive HER2-negative breast cancer, education, close follow-up, dose modification, and prescribing loperamide to accompany the therapy are also important. Above all, be sure to discuss with high-risk patients the opportunity to reduce their risk with appropriate therapy and the importance of therapy adherence in achieving favorable outcomes.
References
1) Dickler MN, Tolaney SM, Rugo HS, Cortés J, Diéras V, Patt D, Wildiers H, Hudis CA, O’Shaughnessy J, Zamora E, Yardley DA, Frenzel M, Koustenis A, Baselga J. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2- Metastatic Breast Cancer. Clin Cancer Res. 2017 Sep 1;23(17):5218-5224. doi: 10.1158/1078-0432.CCR-17-0754. Epub 2017 May 22. Erratum in: Clin Cancer Res. 2018 Nov 1;24(21):5485. PMID: 28533223; PMCID: PMC5581697.
2) Giordano SH, Franzoi MAB, Temin S, Anders CK, Chandarlapaty S, Crews JR, Kirshner JJ, Krop IE, Lin NU, Morikawa A, Patt DA, Perlmutter J, Ramakrishna N, Davidson NE. Systemic Therapy for Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: ASCO Guideline Update. J Clin Oncol. 2022 Aug 10;40(23):2612-2635. doi: 10.1200/JCO.22.00519. Epub 2022 May 31. PMID: 35640077.
3) Navarro-Yepes J, Kettner NM, Rao X, Bishop CS, Bui TN, Wingate HF, Singareeka Raghavendra A, Wang Y, Wang J, Sahin AA, Meric-Bernstam F, Hunt KK, Damodaran S, Tripathy D, Keyomarsi K. Abemaciclib is effective in palbociclib-resistant hormone receptor-positive metastatic breast cancers. Cancer Res. 2023 Jun 29:CAN-23-0705. doi: 10.1158/0008-5472.CAN-23-0705. Epub ahead of print. PMID: 37384539.
4) Johnston SRD, Toi M, O’Shaughnessy J, Rastogi P, Campone M, Neven P, Huang CS, Huober J, Jaliffe GG, Cicin I, Tolaney SM, Goetz MP, Rugo HS, Senkus E, Testa L, Del Mastro L, Shimizu C, Wei R, Shahir A, Munoz M, San Antonio B, André V, Harbeck N, Martin M; monarchE Committee Members. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023 Jan;24(1):77-90. doi: 10.1016/S1470-2045(22)00694-5. Epub 2022 Dec 6. PMID: 36493792.
5) Groenland SL, Martínez-Chávez A, van Dongen MGJ, Beijnen JH, Schinkel AH, Huitema ADR, Steeghs N. Clinical Pharmacokinetics and Pharmacodynamics of the Cyclin-Dependent Kinase 4 and 6 Inhibitors Palbociclib, Ribociclib, and Abemaciclib. Clin Pharmacokinet. 2020 Dec;59(12):1501-1520. doi: 10.1007/s40262-020-00930-x. PMID: 33029704.
Late Breaking Abstract – ASCO 2023: First Line versus Second Line Use of CDK4/6 Inhibitors in Advanced HR-Positive/HER-Negative Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. It is estimated that approximately 300,590 new cases of breast cancer will be diagnosed in 2023 and about 43,700 individuals will die of the disease, largely due to metastatic recurrence. Breast cancer is the second leading cause of cancer death in women, in the U.S.
About 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors, and HR-positive/HER2-negative breast cancer is the most frequently diagnosed molecular subtype. About 90% of all breast cancers are detected at an early stage, and these patients are often cured with a combination of surgery, radiotherapy, chemotherapy, and hormone therapy. However approximately 20% of patients will experience local recurrence or distant relapse during the first 10 years of treatment. This may be more relevant for those with high risk disease, among whom the risk of recurrence is even greater during the first 2 years while on adjuvant endocrine therapy, due to primary endocrine resistance. More than 75% of the early recurrences are seen at distant sites. Factors associated with high risk of recurrence in HR-positive, HER2-negative early breast cancer include positive nodal status, the number of positive nodes, large tumor size (5 cm or more), and high tumor grade (Grade 3).
Cyclin Dependent Kinases (CDKs) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. CDK 4 and 6 phosphorylate RetinoBlastoma protein (RB), and initiate transition from the G1 phase to the S phase of the cell cycle. RetinoBlastoma protein has antiproliferative and tumor-suppressor activity. Phosphorylation of RB protein nullifies its beneficial activities. CDK4 and CDK6 are activated in HR-positive breast cancer, by binding to D-cyclins in the ER-positive breast cancer cell, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation. The understanding of the role of CDKs in the cell cycle, has paved the way for the development of CDK inhibitors.
It has been shown that CDK4/6 inhibitors in combination with endocrine therapy improves Progression Free Survival (PFS) as well as Overall Survival (OS) when given as initial treatment (first-line) and after prior endocrine monotherapy (second-line), in patients with HR-positive, HER2-negative advanced breast cancer. Treatment guidelines recommend first-line use of CDK4/6 inhibitors along with endocrine therapy, but evidence of superiority of first-line use over second-line based on a head-to-head comparison is lacking.
SONIA is a real-world, randomized, investigator-initiated, nationwide, Phase III trial, conducted to evaluate the efficacy, safety and cost-effectiveness of CDK4/6 inhibitors added to either first or second-line endocrine therapy, in patients with HR-positive, HER2-negative advanced breast cancer, who have received no prior therapy for their advanced disease. In this study, 1050 pre and postmenopausal women (N=1050) with measurable or evaluable disease, who received no prior therapy for advanced breast cancer, were randomized 1:1 to receive first-line treatment with a non-steroidal Aromatase Inhibitor and a CDK4/6 inhibitor, followed upon progression by Fulvestrant (strategy A) or first-line treatment with a non-steroidal Aromatase Inhibitor, followed upon progression by Fulvestrant and CDK4/6 inhibitor (strategy B). Both treatment groups were well balanced. Neoadjuvant/adjuvant therapy was allowed if the disease-free interval after non-steroidal Aromatase Inhibitor therapy was more than 12 months. The choice of CDK4/6 inhibitor was a stratification factor and was left to the discretion of the treating physician. The Primary endpoint was time from randomization to second objective disease progression, as assessed by local investigators, or death (PFS2). Secondary endpoints include Overall Survival (OS), Safety, Quality of Life, and cost-effectiveness.
At a median follow-up was 37.7 months, the median duration of CDK4/6 inhibitor treatment/usage was 24.6 months in the first-line group and 8.1 months in the second-line group. The median PFS with strategy A as expected was significantly longer in the CDK4/6 inhibitor group than in the non-steroidal Aromatase Inhibitor group (24.7 months and 16.1 months respectively, HR=0.59; P<0.0001). However, with regards to PFS2, there was no significant difference between the two treatment groups. The median PFS2 was 31.0 months with strategy A versus 27.8 months with strategy B (HR=0.89; P=0.14) and this similar PFS2 treatment effect was consistent across pre-defined subgroups. There was no significant difference in Overall Survival between the two treatment groups (HR=0.98; P=0.83).
There were more grade 3 or 4 adverse events when CDK4/6 inhibitors were used in the first-line setting and the use of strategy A increased the cost of treatment by an average of $200,000 per patient. Quality of life, as measured by Functional Assessment of Cancer Therapy – Breast (FACT-B) total score, was not different between the 2 arms.
It was concluded that first-line use of CDK4/6 inhibitor along with endocrine therapy does not provide statistically significant and clinically meaningful Progression Free Survival benefit compared to second-line use in women with HR-positive and HER2-negative advanced breast cancer. The authors added that second-line use may thus be a preferred option for the majority of these patients, as the use in first-line prolongs the time on CDK4/6 inhibitors by over 16 months and increases toxicity and cost of treatment. It should however be noted that in this study, patients received single-agent Fulvestrant as second-line treatment which may not be the standard treatment intervention, given the approval of Alpelisib for patients with PIK3CA mutations. Treatment selection based on biomarkers testing therefore is important. Based on the SONIA trial data, offering endocrine therapy alone in the first line setting may not be inappropriate for favorable risk patients without high visceral tumor burden.
Primary outcome analysis of the phase 3 SONIA trial (BOOG 2017-03) on selecting the optimal position of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors for patients with hormone receptor-positive (HR+), HER2-negative (HER2-) advanced breast cancer (ABC). Sonke GS, Van Ommen-Nijhof A, Wortelboer N, et al. DOI: 10.1200/JCO.2023.41.17_suppl. LBA1000 Journal of Clinical Oncology 41, no. 17_suppl (June 10, 2023) LBA1000
