The FDA on June 16, 2020 granted accelerated approval to KEYTRUDA® for the treatment of adult and pediatric patients with unresectable or metastatic Tumor Mutational Burden-High (TMB-H) [10 or more mutations/megabase (mut/Mb)] solid tumors, as determined by an FDA-approved test, that have progressed following prior treatment and who have no satisfactory alternative treatment options. KEYTRUDA® is a product of Merck & Co., Inc.
Tag: General Medical Oncology & Hematology
Proton Based Chemoradiotherapy Significantly Decreases Toxicities without Compromising Efficacy
SUMMARY: Radiation Therapy involves the use of X-Rays, Gamma rays and charged particles for cancer treatment. External Beam Radiation Therapy (EBRT) is most often delivered using a linear accelerator in the form of Photon beams (either X-rays or Gamma rays). Photons have no mass and are packets of energy of an electromagnetic wave. Electrons and Protons are charged particles and Electrons are considered light particles whereas Protons are considered heavy particles. Electron beams are used to irradiate skin and superficial tumors, as they are unable to penetrate deep into the tissues. The different types of External Beam Radiation Treatments include 3-Dimensional Conformal Radiation Therapy (3D-CRT) meant to deliver radiation to very precisely shaped target areas, IMRT or Intensity Modulated Radiation Therapy which allows different areas of a tumor or nearby tissues to receive different doses of radiation, Image Guided Radiation Therapy (IGRT) which allows reduction in the planned volume of tissue to be treated, as changes in a tumor size are noted during treatment, Stereotactic RadioSurgery (SRS) which can deliver one or more high doses of radiation to a small tumor and Stereotactic Body Radiation Therapy (SBRT) or CYBERKNIFE® which is similar to SRS but also takes the normal motion of the body into account while treating malignancies involving the lung and liver.
Proton beams unlike Photons, enter the skin and travel through the tissues and deposit much of their energy at the end of their path (known as the Bragg peak), and deposit less energy along the way. This is unlike Photons which deposit energy all along the path through the tissues and the deposited dose decreases with increasing depth. As a result, with Proton beam therapy, normal tissues are exposed to less radiation compared with Photons. Despite this advantage, tissue heterogeneity such as organ motion, tumor volume changes during treatment can have a significant negative impact on target coverage for Proton beam therapy and can result in damage to the surrounding tissues and potential complications. It is well established that there is significant benefit for Proton beam therapy in certain pediatric malignancies.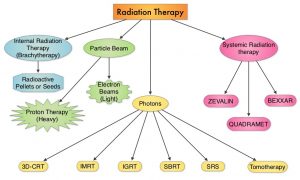
Curative treatment with concurrent chemoradiotherapy is the standard of care for many nonmetastatic, locally advanced cancers. This treatment modality however is associated with substantial morbidity. Proton therapy as component of concurrent chemoradiotherapy might be able to reduce treatment related toxicity and achieve comparable cancer control outcomes, compared with conventional Photon radiotherapy, by reducing the radiation dose to normal tissues. There are however limited data comparing results of Proton chemoradiotherapy with Photon chemoradiotherapy, and Proton therapy remains unproven in this treatment setting. The objective of this study was to assess whether Proton therapy in the setting of concurrent chemoradiotherapy is associated with fewer hospitalizations or other adverse events and similar Disease-free and Overall Survival, compared with concurrent Photon chemoradiotherapy.
In this large single-institution, nonrandomized, comparative effectiveness, retrospective analysis, 1483 adult patients with nonmetastatic, locally advanced cancer, treated with concurrent chemoradiotherapy with curative intent were included. Three hundred ninety-one patients (N=391) received Proton therapy and 1092 patients received Photon therapy. Common tumor sites included head and neck, lung, brain, esophagus/gastric, rectum, and pancreas. The median patient age was 62 years, but patients treated with Protons were significantly older with a median age of 66 years versus 61 years, had less favorable Charlson-Deyo comorbidity scores and had lower integral radiation dose to tissues outside the target. Ninety three percent (93%) of patients in the Photon therapy group were treated with Intensity-Modulated Radiotherapy (IMRT). Baseline ECOG Performance Status was similar between the two treatment cohorts. The Primary end point was 90-day adverse events associated with unplanned hospitalizations (CTCAE version 4 – Grade 3 or more). Secondary end points included ECOG performance status decline during treatment, 90-day adverse events of at least Grade 2 that limit instrumental activities of daily living, and Disease-Free and Overall Survival. The data on adverse events and survival were gathered prospectively.
It was noted that Proton chemoradiotherapy was associated with a significantly lower relative risk of 90-day adverse events of at least Grade 3 (P=0.002), significantly lower relative risk of 90-day adverse events of at least Grade 2 (P=0.006), and decline in Performance Status during treatment (P<0.001). Proton chemoradiotherapy was associated with a two-thirds reduction in adverse events associated with unplanned hospitalizations. At a median follow up of 3.7 years for the Proton cohort and 4.2 years for the Photon cohort, there was no difference in Disease-Free or Overall Survival.
It was concluded from this analysis that in adults with locally advanced cancer, Proton chemoradiotherapy was associated with significantly reduced acute adverse events that caused unplanned hospitalizations, with similar Disease-Free and Overall Survival, compared to Photon therapy.
Comparative Effectiveness of Proton vs Photon Therapy as Part of Concurrent Chemoradiotherapy for Locally Advanced Cancer. Baumann BC, Mitra N, Harton JG, et al. Jama Oncol. 2020;6:237-246.
Antibiotic Use Significantly Decreases Efficacy of Checkpoint Inhibitors in Patients with Advanced Cancer
SUMMARY: The American Cancer Society estimates that in 2020, there will be an estimated 1.8 million new cancer cases diagnosed and 606,520 cancer deaths in the United States. Immunotherapy with Immune Checkpoint Inhibitors (ICIs) has revolutionized cancer care and has become one of the most effective treatment options by improving Overall Response Rate and prolongation of survival across multiple tumor types. These agents target Programmed cell Death protein-1 (PD-1), Programmed cell Death Ligand-1 (PD-L1), Cytotoxic T-Lymphocyte-Associated protein-4 (CTLA-4), and many other important regulators of the immune system. Biomarkers predicting responses to ICI’s include Tumor Mutational Burden (TMB), Mismatch Repair (MMR) status, and Programmed cell Death Ligand 1 (PD‐L1) expression. Other biomarkers such as Tumor Infiltrating Lymphocytes (TILs), TIL‐derived Interferon‐γ, Neutrophil‐to‐Lymphocyte ratio, and peripheral cytokines, have also been proposed as predictors of response. It has been postulated that concomitant medications during therapy with ICIs such as baseline steroid use as well as treatment with antibiotics may negate or lessen the efficacy of ICIs.
Preclinical studies have suggested that immune-based therapies for cancer may have a very complex interplay with the host’s microbiome and there may be a relationship between gut bacteria and immune response to cancer. The crosstalk between microbiota in the gut and the immune system allows for the tolerance of commensal bacteria (normal microflora) and oral food antigens and at the same time enables the immune system to recognize and attack opportunistic bacteria. Immune Checkpoint Inhibitors strongly rely on the influence of the host’s microbiome, and the gut microbial diversity enhances mucosal immunity, dendritic cell function, and antigen presentation. Broad-spectrum antibiotics can potentially alter the bacterial composition and diversity of our gut microbiota, by killing the good bacteria. It has been postulated that this may negate the benefits of immunotherapy and influence treatment outcomes.
This present study was conducted to assess the impact of antibiotic use at the time of ICI treatment, on the outcomes for patients with advanced or metastatic solid tumors. This United Kingdom single institution retrospective analysis included 291 (N=291) patients with advanced cancer, treated with ICI (Melanoma N=179, Non‐Small Cell Lung Cancer N=64, and Renal Cell Carcinoma N=48), who received an ICI agent between January 1, 2015, and April 1, 2017. Antibiotic use (both single and multiple courses as well as prolonged use) during the periods 2 weeks before and 6 weeks after ICI treatment was investigated and data collected. The authors chose this time period, because the potential duration of modification of gut microbiota following antibiotic therapy can vary, for different classes of antibiotics.
Ninety two (N=92) patients in the analyzed cohort had antibiotic therapy during ICI treatment. The use of antibiotics during treatment with ICIs was significantly associated with shorter Progression Free Survival (median PFS 3.1 versus 6.3 months; P=0.003) and Overall Survival (median OS 10.4 versus 21.7 months; P=0.002). Administration of a single course of antibiotics was associated with a non-significant reduction in PFS and OS, whereas patients who had received cumulative courses of antibiotics had significantly worse PFS (median PFS, 2.8 months; P=0.026) and OS (median OS, 6.3 months; P=0.009). Cumulative use of antibiotics was an independent significant prognostic factor for clinical outcomes among patients treated with ICIs.
It was concluded from this large, multivariate analysis that antibiotic use is an independent negative predictor of PFS and OS in patients with advanced cancer treated with Immune Checkpoint Inhibitors, with worse treatment outcomes among patients who had received multiple or prolonged courses of antibiotics. The authors added that this is the first study to suggest an adverse effect of cumulative antibiotic use, in patients receiving treatment with Immune Checkpoint Inhibitors for advanced cancer. Cumulative Antibiotic Use Significantly Decreases Efficacy of Checkpoint Inhibitors in Patients with Advanced Cancer. Tinsley N, Zhou C, Tan G, et al. Oncologist. 2020;25:55-63.
Blood-Based Screening Test Identifies Gastrointestinal Cancers
SUMMARY: It is estimated that cancers of the esophagus, stomach, pancreas, gallbladder, liver, bile duct, colon and rectum account for approximately 17% of incident cancer diagnoses and 26% of cancer-related deaths in the US. There are currently no screening tests available for cancers of the gallbladder, bile duct, and pancreatic cancer. Although screening tests do exist for other types of GI malignancies such as colorectal and stomach cancer, many of them are invasive. Further, when GI malignancies are diagnosed, they are frequently at advanced stages and are more difficult to treat.
A noninvasive, liquid biopsy assay based on circulating tumor DNA (ctDNA) has the potential to detect cancer in early stages among asymptomatic individuals. ctDNA refers to DNA fragments that are shed into the bloodstream by cancer cells after apoptosis or necrosis. ctDNA can detect almost all molecular alterations present in cancer cells and genotyping circulating cell-free tumor DNA (cfDNA) in the plasma can potentially overcome the shortcomings of repeat biopsies and tissue genotyping, allowing the detection of many more targetable gene mutations, thus resulting in better evaluation of the tumor genome landscape. The proportion of cfDNA that originates from a tumor depends on the anatomic location, tumor burden and cell turnover. cfDNA also allows real-time monitoring for treatment response and resistance.
The Cancer Genome Atlas (TCGA), a landmark cancer genomics program, is a joint effort between the National Cancer Institute and the National Human Genome Research Institute. This program began in 2006 and has molecularly characterized over 20,000 primary cancers and matched normal samples, across 33 different cancer types. After 12 years and contributions from over 11,000 patients, TCGA has deepened our understanding of the molecular basis of cancer, changed the way cancer patients are managed in the clinic, established a rich genomics data resource for the research community, and helped advance health and science technologies.
The Circulating Cell-Free Genome Atlas (CCGA) is a prospective, multi-center, case-control, observational study with longitudinal follow up, and is the largest study ever initiated, to develop a noninvasive, liquid biopsy assay for early cancer detection based on cell-free DNA (cfDNA). This study completed enrollment of approximately 15,000 participants with and without cancer (56% with more than 20 tumor types and all clinical stages), across 142 sites in the US and Canada. The purpose of this study is to collect biological samples from patients with a new diagnosis of cancer (blood and tumor tissue) and from individuals who do not have a diagnosis of cancer (blood), in order to characterize the population heterogeneity in cancer and non-cancer participants, and to develop models for distinguishing cancer from non-cancer. The principal goal is to develop a noninvasive cancer detection assay and the CCGA was designed to characterize the landscape of genomic cancer signals in the blood and to detect and validate GRAIL, Inc.’s multi-cancer early detection blood test through three pre-planned sub-studies.
GRAIL, Inc., is a healthcare company focused on the early detection of cancer by using the power of Next-Generation Sequencing, population-scale clinical studies, and state-of-the-art computer science and data science to enhance the scientific understanding of cancer biology, and to develop its multi-cancer early detection blood test. GRAIL’s high efficiency methylation technology preferentially targets the most informative regions of the genome, and is designed to use its proprietary database and machine-learning algorithms to both detect the presence of cancer and identify the tumor’s Tissue of Origin. GRAIL’s sequencing database of cancer and non-cancer methylation signatures is believed to be the largest of its kind, and covers approximately 30 million methylation sites across the genome, with more than 20 cancer types across stages represented within the database.
Previously reported data from the first sub-study of CCGA showed GRAIL’s prototype technology could detect the presence of multiple deadly cancer types with a low rate of false positive results (high specificity). In this analysis blood samples from 166 participants who had a cancer diagnosis at the time of enrollment were evaluated, and cancer was detected using the methylation technology. Results showed that GRAIL’s prototype technology correctly identified the tumor’s Tissue of Origin in 87% of the blood samples evaluated (N=144/166), including 96% of breast cancer cases (N=22/23), 88% of lung cancer cases (N=29/33), 90% of liver cancer cases (N=9/10) and 100% of pancreatic cancer cases (N=17/17).
GRAIL has since selected methylation as its preferred approach and evaluated its refined methylation blood test in the second pre-planned sub-study of CCGA. It was determined that whole-genome bisulfite sequencing for DNA methylation was the most effective approach for early cancer detection. DNA methylation is a natural epigenetic mechanism used by cells to regulate gene expression with some regions of hypermethylation and some regions of hypomethylation, and is a chemical modification to DNA, that can change how a gene’s function is carried out by the body without changing the order of the DNA bases. In cancer, abnormal methylation patterns and the resulting changes in gene expression can contribute to tumor growth (hypermethylation can cause tumor-suppressor genes to be inactivated). Methylation patterns or signatures, are unique to the tumor DNA, enabling tumor detection and localization, but are not of value when it comes to precision therapies. This is unlike mutations and copy number changes, which can be detected in white blood cells in individuals without cancer as well, leading to false-positives.
The researches in this second substudy reported the performance of methylation-based cfDNA early multi-cancer detection test, for GastroIntestinal (GI) tract cancers, and also provided data from individuals without known cancer (non-cancer controls). To test the current assay, the second substudy included approximately 4,500 individuals, both with and without cancer, who were split into a training cohort and a validation cohort. Of the 2,185 patients with newly-diagnosed cancer in the second substudy, 447 patients were diagnosed with GI malignancies. Plasma cfDNA was subjected to targeted methylation analysis to develop an algorithm that could identify the presence or absence of cancer, as well as the Tissue of Origin of the cancer. The GI malignancies included Esophagus/Stomach (N=67), Pancreas/Gallbladder/Extrahepatic bile duct (N=95), Liver/Intrahepatic bile duct (N=29), and Colon/Rectum (N=121). To minimize the likelihood of false-positives, the targeted methylation assay was pre-set to yield greater than 99% specificity.
The test showed a sensitivity level of approximately 82% for detecting GI cancers of all stages in the independent validation set. The predicted Tissue of Origin accuracy across all GI cancers was 92%.
It was concluded that this assay performed using a single noninvasive blood sample, has the potential to diagnose a variety of gastrointestinal cancers earlier, when they are more treatable, with good sensitivity and with a low rate of false positives. Performance of a blood-based test for the detection of multiple cancer types. Wolpin BM, Richards DA, Cohn AL, et al. J Clin Oncol. 2020;38(suppl 4; abstr 283).
Medication-Related Osteonecrosis of the Jaw: MASCC/ISOO/ASCO Clinical Practice Guideline Summary
BMAs that have been linked with MRONJ principally include bisphosphonates such as Zoledronic acid and Pamidronate and Rank Ligand inhibitor, Denosumab. BMAs are an integral part of cancer management and have essential roles in supportive oncology for the treatment of hypercalcemia of malignancy and bone metastases, and prevention of skeletal-related events such as pathologic fractures and reduce the need for radiation or surgical intervention. BMAs disrupt the bone remodeling cycle by reducing osteoclast survival and function.
The incidence of MRONJ in the osteoporosis patient population is very low and majority of the MRONJ cases occur in the oncology patient population receiving high doses of BMAs and prevalence has been estimated to be as high as 18.6%. The incidence in cancer patients appears to be related to dose and duration of exposure to BMAs. Bisphosphonates-related ONJ occurs after a mean IV administration of 33 months in cancer patients, whereas Denosumab-related ONJ occurs early after treatment, independent of the number of previous administrations. Risk factors for ONJ while on BMAs include smoking, poor oral hygiene, ill-fitting dentures, invasive dental procedures, and uncontrolled diabetes. Chemotherapeutic agents such as angiogenesis inhibitors, Tyrosine Kinase Inhibitors, mTOR inhibitors and immunotherapeutic agents have also been implicated.
The expert panel including representatives from ASCO, the Multinational Association of Supportive Care in Cancer, and the International Society of Oral Oncology outlined best practice recommendations for the prevention and management of MRONJ in patients with cancer who receive BMAs for oncologic indications, following a systematic review of the medical literature. Given the paucity of high-quality evidence, a majority of the recommendations are based on consensus using ASCO’s formal consensus process. The guideline does not address BMAs used for osteoporosis, which are administered at a lower dose and carry a lower risk for MRONJ.
Medication-Related Osteonecrosis of the Jaw: MASCC/ISOO/ASCO Clinical Practice Guideline Summary
Guideline Question: What are the recommended best practices for preventing and managing medication-related osteonecrosis of the jaw (MRONJ) in patients with cancer?
Target Population: Adult patients with cancer who are receiving Bone-Modifying Agents (BMAs) for any oncologic indication.
Target Audience: Oncologists and other physicians, dentists, dental specialists, oncology nurses, clinical researchers, oncology pharmacists, advanced practitioners, and patients with cancer.
Recommendations:
Clinical Question 1. What is the preferred terminology and definition for OsteoNecrosis of the Jaw (maxilla and mandible) associated with pharmacologic therapies in oncology patients?
Recommendation 1.1. It is recommended that the term Medication-Related OsteoNecrosis of the Jaw (MRONJ) be used when referring to bone necrosis associated with pharmacologic therapies.
Recommendation 1.2. Clinicians should confirm the presence of all three of the following criteria to establish a diagnosis of MRONJ – a) Current or previous treatment with a BMA or angiogenic inhibitor b) Exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region and that has persisted for longer than 8 weeks c) No history of radiation therapy to the jaws or metastatic disease to the jaws
Clinical Question 2. What steps should be taken to reduce the risk of MRONJ?
Recommendation 2.1. (Coordination of care.) For patients with cancer who are scheduled to receive a BMA in a non-urgent setting, oral care assessment (including a comprehensive dental, periodontal, and oral radiographic exam when feasible to do so) should be undertaken before initiating therapy. On the basis of the assessment, a dental care plan should be developed and implemented. The care plan should be coordinated between the dentist and the oncologist to ensure that medically necessary dental procedures are undertaken before initiation of the BMA. Follow-up by the dentist should then be performed on a routine schedule (eg, every 6 months) once therapy with a BMA has commenced.
Recommendation 2.2. (Modifiable risk factors.) Members of the multidisciplinary team should address modifiable risk factors for MRONJ with the patient as early as possible. These risk factors include poor oral health, invasive dental procedures, ill-fitting dentures, uncontrolled diabetes mellitus, and tobacco use.
Recommendation 2.3. (Elective dentoalveolar surgery.) Elective dentoalveolar surgical procedures (eg, non–medically necessary extractions, alveoloplasties, and implants) should not be performed during active therapy with a BMA at an oncologic dose. Exceptions may be considered when a dental specialist with expertise in prevention and treatment of MRONJ has reviewed the benefits and risks of the proposed invasive procedure with the patient and the oncology team.
Recommendation 2.4. (Dentoalveolar surgery follow-up.) If dentoalveolar surgery is performed, patients should be evaluated by the dental specialist on a systematic and frequently scheduled basis (eg, every 6 to 8 weeks) until full mucosal coverage of the surgical site has occurred. Communication with the oncologist regarding status of healing is encouraged, particularly when considering future use of BMA.
Recommendation 2.5. (Temporary discontinuation of BMAs before dentoalveolar surgery.) For patients with cancer who are receiving a BMA at an oncologic dose, there is insufficient evidence to support or refute the need for discontinuation of the BMA before dentoalveolar surgery. Administration of the BMA may be deferred at the discretion of the treating physician, in conjunction with discussion with the patient and the oral health provider.
Clinical Question 3. How should MRONJ be staged?
Recommendation 3.1. A well-established staging system should be used to quantify the severity and extent of MRONJ and to guide management decisions. Options include the 2014 American Association of Oral and Maxillofacial Surgeons staging system, the Common Terminology Criteria for Adverse Events version 5.0, and the 2017 International Task Force on Osteonecrosis of the Jaw staging system for MRONJ. The same system should be used throughout the patient’s MRONJ course of care. Diagnostic imaging may be used as an adjunct to these staging systems.
Recommendation 3.2. Optimally, staging should be performed by a clinician experienced with the management of MRONJ
Clinical Question 4. How should MRONJ be managed?
Recommendation 4.1. (Initial treatment of MRONJ.) Conservative measures compose the initial approach to treatment of MRONJ. Conservative measures may include antimicrobial mouth rinses, antibiotics if clinically indicated, effective oral hygiene, and conservative surgical interventions (eg, removal of a superficial bone spicule).
Recommendation 4.2. (Treatment of refractory MRONJ.) Aggressive surgical interventions (eg, mucosal flap elevation, block resection of necrotic bone, soft tissue closure) may be used if MRONJ results in persistent symptoms or affects function despite initial conservative treatment. Aggressive surgical intervention is not recommended for asymptomatic bone exposure. In advance of the aggressive surgical intervention, the multidisciplinary care team and the patient should thoroughly discuss the risks and benefits of the proposed intervention.
Clinical Question 5. Should BMAs be temporarily discontinued after a diagnosis of MRONJ has been established?
Recommendation 5. For patients diagnosed with MRONJ while being treated with BMAs, there is insufficient evidence to support or refute the discontinuation of the BMAs. Administration of the BMA may be deferred at the discretion of the treating physician, in conjunction with discussion with the patient and the oral health provider.
Clinical Question 6. What outcome measures should be used in clinical practice to describe the response of the MRONJ lesion to treatment?
Recommendation 6. During the course of MRONJ treatment, the dentist or dental specialist should communicate with the medical oncologist the objective and subjective status of the lesion (ie, resolved, improving, stable, or progressive). The clinical course of MRONJ may impact local and/or systemic treatment decisions with respect to cessation or recommencement of BMAs.
The Multinational Association of Supportive Care in Cancer, International Society of Oral Oncology, and ASCO believe that cancer clinical trials are vital to inform medical decisions and improve cancer care, and that all patients should have the opportunity to participate.
Medication-Related Osteonecrosis of the Jaw: MASCC/ISOO/ASCO Clinical Practice Guideline Summary. Shapiro CL, Yarom N, Peterson DE, et al. J Oncol Practice 2019;15: 603-606.
REBLOZYL® (Luspatercept-aamt)
The FDA on November 8, 2019 approved REBLOZYL® for treatment of anemia in adult patients with beta Thalassemia who require regular red blood cell transfusions. REBLOZYL® is a product of Celgene Corp.
GIVLAARI® (Givosiran)
The FDA on November 20, 2019 approved GIVLAARI® for adults with Acute Hepatic Porphyria (AHP). GIVLAARI® is a product of Alnylam Pharmaceuticals, Inc.
Antibiotic Treatment Prior to Immune Checkpoint Inhibitor Therapy has a Detrimental Effect on Response Rates and Overall Survival
SUMMARY: The American Cancer Society estimates that in 2019, there will be an estimated 1,762,450 new cancer cases diagnosed and 606,880 cancer deaths in the United States. Immunotherapy with Immune Checkpoint Inhibitors (ICIs) has revolutionized cancer care and has become one of the most effective treatment options by improving Overall Response Rate and prolongation of survival across multiple tumor types. These agents target Programmed cell death protein-1 (PD-1), Programmed cell death ligand-1 (PD-L1), Cytotoxic T-Lymphocyte-Associated protein-4 (CTLA-4), and many other important regulators of the immune system.
Preclinical studies have suggested that immune-based therapies for cancer may have a very complex interplay with the host’s microbiome and there may be a relationship between gut bacteria and immune response to cancer. The crosstalk between microbiota in the gut and the immune system allows for the tolerance of commensal bacteria (normal microflora) and oral food antigens and at the same time enables the immune system to recognize and attack opportunistic bacteria. Immune Checkpoint Inhibitors strongly rely on the influence of the host’s microbiome, and the gut microbial diversity enhances mucosal immunity, dendritic cell function, and antigen presentation. Broad-spectrum antibiotics can alter the bacterial composition and bacterial diversity of our gut, by killing the good bacteria. It has been postulated that this may negate the benefits of immunotherapy and influence treatment outcomes.
The authors conducted this study to determine whether there was an association between antibiotic therapy administered prior to or concurrently with ICI therapy and Overall Survival (OS) and Response Rates, in patients with cancer, treated with ICIs in routine clinical practice. In this prospective, multicenter cohort study, 196 patients with cancer who received ICI therapy were recruited at two tertiary care centers between January 2015 and April 2018. Majority of enrolled patients had Non-Small Cell Lung Cancer (N=119), but patients with Melanoma (N=38) as well as Urologic and Head and Neck cancers (N=39) were also included in the analysis. The median age was 68 years, and majority of patients had metastatic disease at the time of treatment initiation with ICIs and 96% of patients received anti-PD-1/PD-L1 therapy alone. Broad spectrum antibiotics up to 30 days prior to commencement of ICI qualified as prior antibiotic exposure whereas concurrent treatment with antibiotics was defined as antibiotic treatment from the first day of ICI treatment until cessation. Beta-lactams were the most commonly prescribed antibiotic class, and were given as a single course for less than 7 days. When antibiotics were administered concurrently with ICIs, patients tended to be treated longer and with multiple courses. The common indication for both prior and concurrent antibiotic treatment was respiratory infections, and 15% of patients received antibiotic therapy prior to ICI therapy, whereas 35% of patients received antibiotics concurrently with ICIs. The Primary endpoint was Overall Survival (OS), calculated from the time of ICI therapy commencement and radiologic response to treatment, with disease refractory to ICI therapy defined as progressive disease 6-8 weeks after the first ICI dose, without evidence of pseudoprogression.
In this analysis, antibiotic treatment prior to ICI therapy had a significant adverse effect on Overall Survival, with a median survival of only 2 months for those who received prior antibiotic treatment versus 26 months for antibiotic-naive patients (HR=7.4; P<0.001). Further, patients who had received prior antibiotic treatment had a higher likelihood of primary refractoriness to ICIs, compared to those who did not receive antibiotics (81% versus 44% (P<0.001). The poor OS outcomes when patients received antibiotic treatment prior to ICI therapy were noted, irrespective of tumor site (OS in NSCLC 26 vs 2.5 months, P<0.001, OS in Melanoma 14 vs 3.9 months, P<0.001, OS in other tumors 11 vs 1.1 months, P <0.001). Multivariate analyses confirmed that prior antibiotic therapy and response to ICI therapy were associated with OS, independent of tumor site, disease burden, and performance status. Antibiotic treatment administered concurrently with ICIs however, was not associated with worse Overall Survival.
It was concluded that treatment with antibiotics prior to therapy with Immune Checkpoint Inhibitors in routine clinical practice, is associated with a worse treatment response and Overall Survival in unselected group of patients. This study suggests that timing of antibiotic exposure may be crucial and the authors recommend that studies are urgently required to investigate antibiotic-mediated alterations of gut microbiota as a determinant of poorer outcomes, following treatment with Immune Checkpoint Inhibitors. Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. Pinato DJ, Howlett S, Ottaviani D, et al. JAMA Oncol. 2019, Sep 12. doi: 10.1001/jamaoncol.2019.2785. [Epub ahead of print]
Liquid Biopsy DNA Methylation Assay Highly Specific for Cancer Detection and Prognosis
SUMMARY: Screening both healthy and high-risk populations with a peripheral blood sample (liquid biopsy) has the potential to detect cancer at an early stage, with an increased opportunity to offer curative therapies. Screening assays for cancer should be highly specific with a low rate of false-positive results and overdiagnosis. Analysis of cell-free DNA (cfDNA) with a Liquid Biopsy is presently approved to select EGFR targeted therapies (cobas EGFR mutation test), in patients with advanced Non Small Cell Lung Cancer. However, the role of cell-free DNA analysis for early detection of cancer is not well established.
The Cancer Genome Atlas (TCGA), a landmark cancer genomics program, is a joint effort between the National Cancer Institute and the National Human Genome Research Institute. This program began in 2006 and has molecularly characterized over 20,000 primary cancers and matched normal samples, across 33 different cancer types. After 12 years and contributions from over 11,000 patients, TCGA has deepened our understanding of the molecular basis of cancer, changed the way cancer patients are managed in the clinic, established a rich genomics data resource for the research community and helped advance health and science technologies.
The Circulating Cell-Free Genome Atlas (CCGA) is a prospective, multi-center, observational study and is the largest study ever initiated, to develop a noninvasive, liquid biopsy assay for early cancer detection, based on cell-free DNA (cfDNA). This study completed enrollment of approximately 15,000 participants with and without cancer (56% with 20 tumor types and all clinical stages), across 142 sites in the US and Canada. The principal goal is to develop a noninvasive cancer detection assay and the CCGA was designed to characterize the landscape of genomic cancer signals in the blood and to detect and validate GRAIL’s multi-cancer early detection blood test through three pre-planned sub-studies. The authors in 2018 previously reported that it is possible to detect early-stage lung cancer, with a high degree of specificity, from a simple blood test, using targeted sequencing and whole-genome sequencing. In this substudy, liquid biopsy could accurately detect over 40% of early-stage lung cancers (Stage I-IIIA), with 98% specificity. It was determined that whole-genome bisulfite sequencing for DNA methylation was the most effective approach for early cancer detection. 
DNA methylation is a natural epigenetic mechanism used by cells to regulate gene expression with some regions of hypermethylation and some regions of hypomethylation, and is a chemical modification to DNA. In cancer, abnormal methylation patterns and the resulting changes in gene expression can contribute to tumor growth (hypermethylation can cause tumor-suppressor genes to be inactivated). Methylation patterns, are unique to the tumor DNA, enabling tumor detection and localization but are not of value when it comes to precision therapies. This is unlike mutations and copy number changes, which can be detected in white blood cells in individuals without cancer as well, leading to false-positives.
In two separate presentations, the authors in this present sub-study reported the results for patients with more than 20 cancer subtypes across all stages and evaluated the prognostic significance of detecting abnormal patterns of cfDNA methylation by whole-genome bisulfite sequencing (WGBS) assay. The goal of targeted methylation assay was to detect both early and advanced disease cancers, and improve clinical outcomes
Liu, MC, et al. reported outcomes for 2,301 participants (1422 had cancer and 879 did not) with more than 20 cancer types (12 prespecified and high-risk cancers included Lung, HR negative Breast, Colorectal, Anorectal, Esophageal, Gastric, Liver, Pancreatic, Head and Neck, Ovary, Myeloma and Lymphoid neoplasms) across all stages. The 12 prespecified cancers account for two thirds of all cancer deaths in the US. At 99% specificity, the sensitivity for these 12 high-risk cancers ranged from 59-86% at early stages (stages I–III). For all 20 cancer types, the overall detection rate across all stages was 55%. Additionally, a Tissue of Origin result was provided for 94% of all cancers detected and of these, the assay correctly identified the Tissue of Origin in 90% of cases, which the authors commented is critical for guiding efficient downstream workup for a positive signal.
Oxnard GR, et al. performed an exploratory longitudinal analysis and reported the results of the Overall Survival of 1,320 participants with more than 20 cancer types in this substudy, thereby evaluating the prognostic significance of detection by this assay. Across all stages of disease, cancers detected by cfDNA whole-genome bisulfite sequencing for DNA methylation were associated with significantly worse survival than those not detected by the blood test. The 2-year Overall Survival was less than 50% among patients whose cancers were detected by the assay compared with 2-year OS of over 90% for those whose cancers were not detected by this assay. The poor prognostic ability of this assay was seen in both cancers that presented with symptoms and those found via screening suggesting that DNA–based detection with this methylation assay may be an indicator of prognosis. In multivariate analysis, cancers detected by this assay had double the risk of death (HR=2.6; P< 0.001) when accounting for clinical stage, cancer type, histologic grade, age, sex, and method of diagnosis and also had comparable prognostic significance to clinical stage (P <0.001).
It was concluded from these two presentations that cfDNA test based on the presence of DNA methylation is highly specific at detecting high-risk malignancies, with very high accuracy for identifying the tissue of origin, and may also have prognostic value.
Genome-wide Cell-free DNA (cfDNA) Methylation Signatures and Effect on Tissue of Origin (TOO) Performance. Liu MC, Jamshidi A, Venn O, et al. 2019 ASCO Annual Meeting. Abstract 3049. Presented June 1, 2019.
Prognostic significance of blood-based cancer detection in plasma cell-free DNA (cfDNA): Evaluating risk of overdiagnosis. Oxnard GR, Chen X, Fung ET, et al. 2019 ASCO Annual Meeting. Abstract 1545. Presented June 3, 2019.
