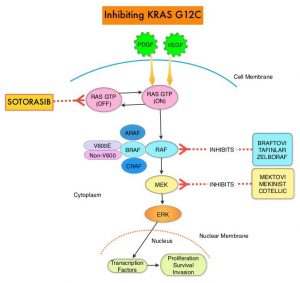
The NSCLC Landscape Has Evolved Significantly Due Largely to the Growing Number of Actionable Mutations1
Despite advancements in standard-of-care, advanced non-small cell lung cancer (NSCLC) continues to burden patients, with poor survival outcomes.2,3 NSCLC has been identified as the leading cause of cancer death worldwide with an estimated 1.8 million deaths in 2020.2 As the number of targeted therapies and approved companion diagnostics continues to grow, mortality and survival rates have begun to improve.3 With the addition of KRAS G12C, there are 9 actionable molecular biomarkers (as of February 2022) and more than 20 targeted therapies approved for use in advanced NSCLC.1,4 Guidelines recommend biomarker testing for all eligible patients at diagnosis of advanced NSCLC regardless of characteristics such as smoking history, race, or histology.5,6 Unfortunately, real-world evidence shows that far too many patients fail to receive the comprehensive biomarker testing.7
Adherence to Guidelines Can Improve Patient Outcomes8
As targeted therapies are approved, guidelines continue to update their recommendations on biomarker testing.5 As of March 2022, NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for NSCLC recommend broad molecular testing of actionable and emerging biomarkers for eligible patients with advanced or metastatic NSCLC (Figure 1).5 Similarly, the American Society of Clinical Oncology (ASCO) endorsed the 2018 College of American Pathologists (CAP)/International Association for the Study of Lung Cancer (IASLC)/Association for Molecular Pathology (AMP) guidelines, recommending comprehensive cancer panel testing for genetic biomarkers.9,10
Figure 1: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for NSCLC5,*,†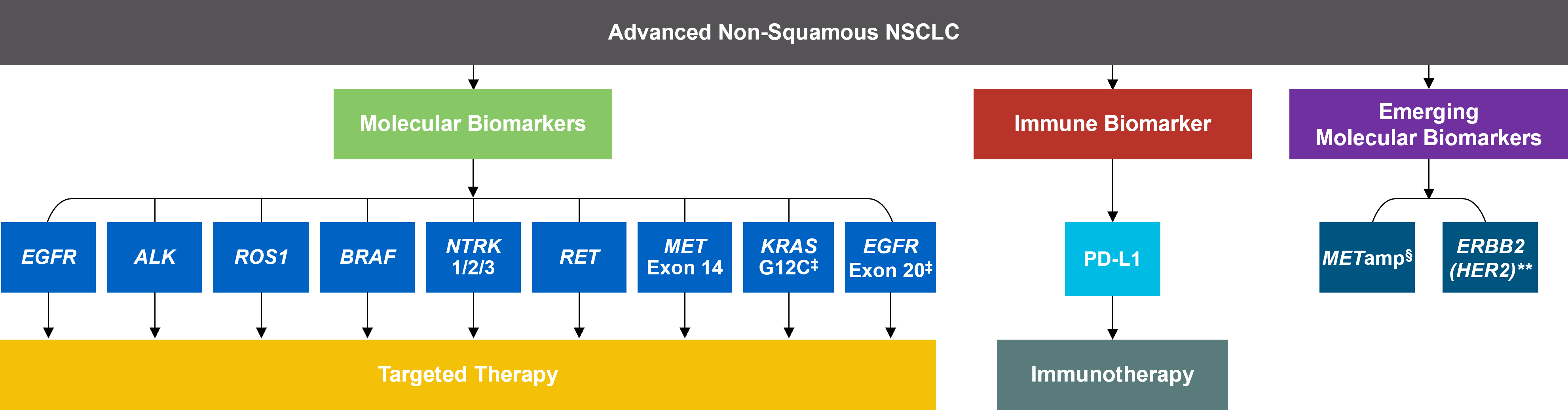 *The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for NSCLC provide recommendations for certain individual biomarkers that should be tested and recommend testing techniques but do not endorse any specific commercially available biomarker assays or commercial laboratories.5†The NCCN Guidelines® for NSCLC recommend broad molecular testing to identify rare driver variants for which targeted therapies may be available to ensure patients receive the most appropriate treatment.5‡KRAS G12C and EGFR exon 20 mutations are used to determine subsequent (ie, second-line and beyond) therapy using targeted agents or other novel agents.5 §The definition of high-level MET amplification is evolving and may differ according to the assay used for testing. For NGS-based results, a copy number greater than 10 is consistent with high-level MET amplification.5 **For oncogenic or likely oncogenic HER2 mutations, refer to definitions at oncokb.org.5
*The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for NSCLC provide recommendations for certain individual biomarkers that should be tested and recommend testing techniques but do not endorse any specific commercially available biomarker assays or commercial laboratories.5†The NCCN Guidelines® for NSCLC recommend broad molecular testing to identify rare driver variants for which targeted therapies may be available to ensure patients receive the most appropriate treatment.5‡KRAS G12C and EGFR exon 20 mutations are used to determine subsequent (ie, second-line and beyond) therapy using targeted agents or other novel agents.5 §The definition of high-level MET amplification is evolving and may differ according to the assay used for testing. For NGS-based results, a copy number greater than 10 is consistent with high-level MET amplification.5 **For oncogenic or likely oncogenic HER2 mutations, refer to definitions at oncokb.org.5
Although adherence to guideline-recommended biomarker testing is associated with improved patient outcomes, real-world EMR data reveals suboptimal biomarker testing rates.8,11 In a retrospective study,†† 81% of patients with metastatic NSCLC did not receive testing for ALK, EGFR, ROS1, and BRAF before initiation of first-line treatment, despite availability of targeted therapies.11 Moreover, only 28% of patients received testing for all four genetic biomarkers and PD-L1 during the study period.11 In another retrospective study, less than 50% of patients with metastatic NSCLC received testing for all five biomarkers (EGFR, ALK, ROS1, BRAF, PD-L1) (Figure 2).7
Beyond the underutilization of biomarker testing, there remains an even greater need to increase broad molecular testing among racial and ethnic minority groups in the US.12,13 In one retrospective study, Black/African American patients with advanced NSCLC had significantly lower rates of testing with NGS assays (39.8%) compared with White patients (50.1%) (Figure 3).12
††A retrospective study assessing real-world biomarker testing patterns in patients with de novo mNSCLC (N=2,257) in the community oncology setting using the US Oncology Network electronic health records between January 1st, 2017 and September 31st, 2019 with follow-up through December 31st, 2019.11
Figure 2: MYLUNG Consortium™ EMR Analysis of Patients With Metastatic NSCLC7,‡‡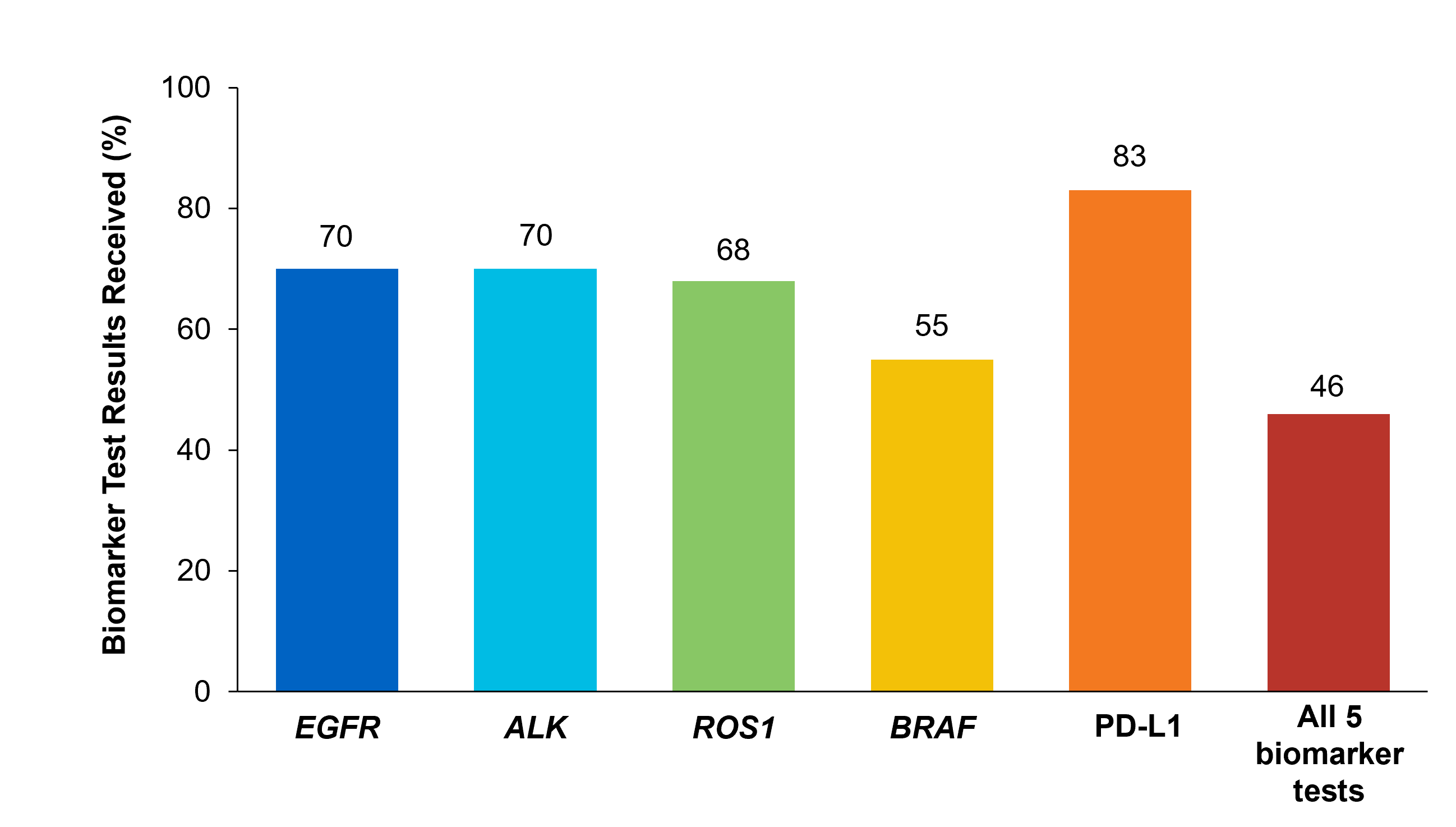 ‡‡A retrospective, observational study assessing real-world biomarker testing patterns in patients with metastatic NSCLC(N=3,474) from community oncology practices within the US Oncology Network community practices between 2018 and 2020.7
‡‡A retrospective, observational study assessing real-world biomarker testing patterns in patients with metastatic NSCLC(N=3,474) from community oncology practices within the US Oncology Network community practices between 2018 and 2020.7
Figure 3: EMR Analysis of Biomarker Testing in Patients With Advanced/Metastatic NSCLC12,§§
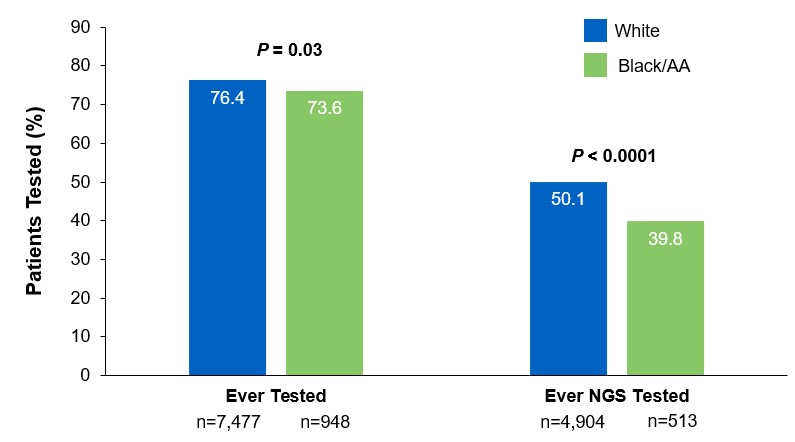 §§From a retrospective cohort study of patients with advanced/metastatic: NSCLC (N=14,768) from ~800 sites of care identified via the Flatiron Electronic Health Record Database between 2017 and 2020. Of this study cohort, patients included White (n=9,793), Black/African American (n=1,288), and non-squamous NSCLC (n=10,333).
§§From a retrospective cohort study of patients with advanced/metastatic: NSCLC (N=14,768) from ~800 sites of care identified via the Flatiron Electronic Health Record Database between 2017 and 2020. Of this study cohort, patients included White (n=9,793), Black/African American (n=1,288), and non-squamous NSCLC (n=10,333).
Collectively, these findings highlight the disparity in proactive disease management across different patient populations.7,11,12
Considerations Across the Biomarker Testing Journey
There are several different methods in which eligible patients can be tested for actionable genetic alterations, each with unique considerations as indicated below (Figure 4).
Figure 4: Comparing Biomarker Testing Methods and Sample Types
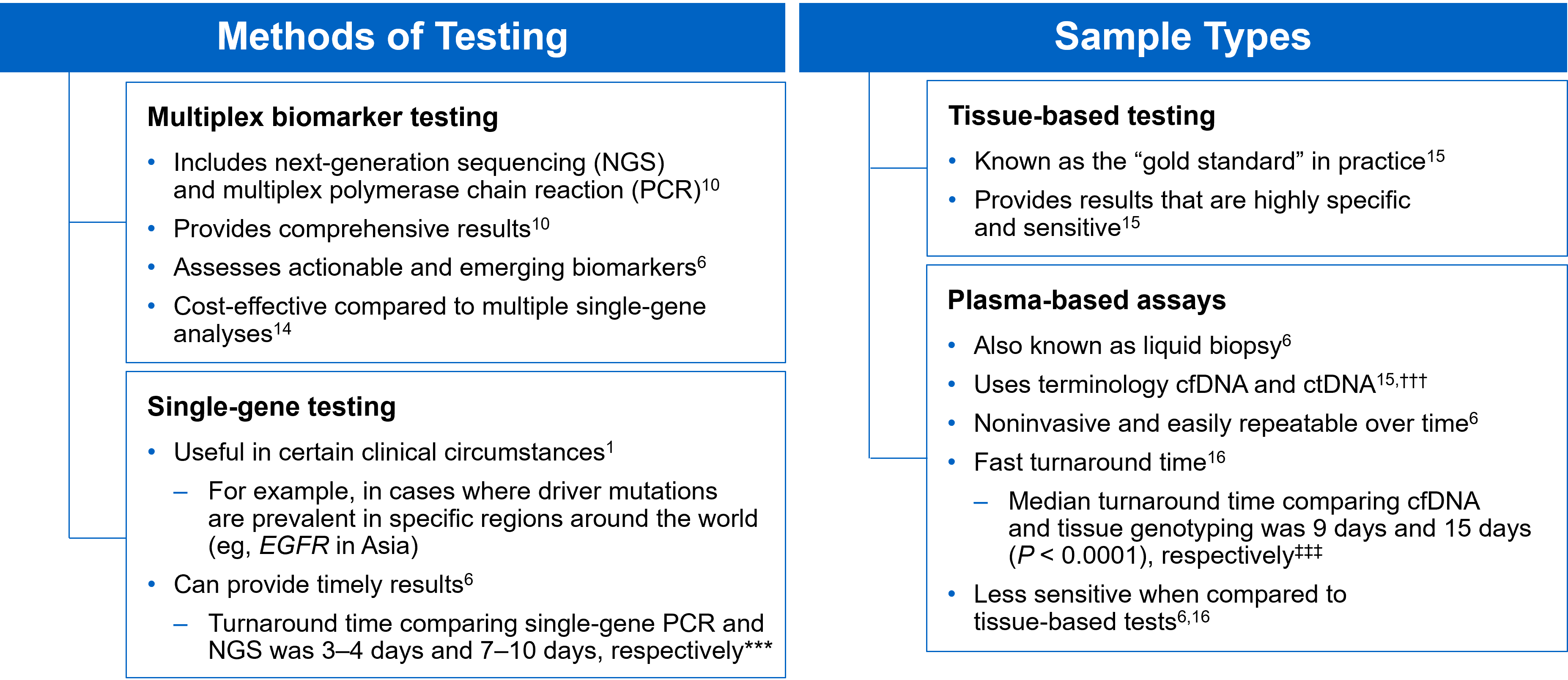 ***Data from a review of common molecular assays for biomarker testing that analyzed common detected variants, sensitivities, and turnaround time.6 †††cfDNA refers to all circulating DNA (largely non-malignant), while ctDNA refers to the tumor-related component of cfDNA.15 ‡‡‡Data from a prospective study that enrolled patients with previously untreated metastatic NSCLC undergoing SOC tissue genotyping and comprehensive cfDNA analysis, with turnaround time defined as the number of days between test order date and the retrieval of test results.16
***Data from a review of common molecular assays for biomarker testing that analyzed common detected variants, sensitivities, and turnaround time.6 †††cfDNA refers to all circulating DNA (largely non-malignant), while ctDNA refers to the tumor-related component of cfDNA.15 ‡‡‡Data from a prospective study that enrolled patients with previously untreated metastatic NSCLC undergoing SOC tissue genotyping and comprehensive cfDNA analysis, with turnaround time defined as the number of days between test order date and the retrieval of test results.16
While tissue biopsy remains the “gold standard” in NSCLC, it may not be feasible (insufficient tissue) or pragmatic (urgent need to begin treatment) in all patients.17 Plasma ctDNA demonstrates complementary results to tissue-based assays and can be considered a valid tool for genotyping of newly diagnosed patients with advanced NSCLC.15 In a prospective study of patients with previously untreated, non-squamous metastatic NSCLC from 2016 to 2018, guideline-recommended biomarkers with FDA-approved therapies (EGFR Exon 19 deletion and L858R, ALK fusion, ROS1 fusion, BRAF V600E) showed ≥ 98.2% concordance between tissue and liquid-based testing.16 While concordance is high for any single test, high concordance for full panels will be required for liquid biopsies to become standard; additionally, negative results on liquid biopsy still require validation with tissue testing.16,17
Liquid biopsy may offer improvements in sample acquisition and small tissue samples and provides less invasive procedures and shortened turnaround times.17 Other considerations for maximizing the tissue journey include the use of comprehensive testing, rapid on-site evaluation (ROSE), and implementing reflex testing protocols with the help of a multidisciplinary team (MDT).17
Delays in Biomarker Testing Results May Impact Treatment Plan Decisions18
Longer turnaround times for molecular testing compared with turnaround times for PD-L1 testing by IHC may result in the initiation of immunotherapy before molecular testing results are received.18 Waiting for complete biomarker test results prior to initiating therapy can allow doctors to make the most informed decisions surrounding a patient’s treatment journey.18
Consider Comprehensive Biomarker Testing as an Important Part of Your Treatment Plan8
As the NSCLC landscape continues to progress with the increasing number of actionable biomarkers, there is a growing need for proactive and comprehensive molecular testing.7,17 Although real-world data has shown significant underuse of biomarker testing, rates can be improved with diligent observation of expanding guidelines and recommendations by expert panels and associations.7,8 In the coming years, clinicians may consider evolving institutional protocols, including enabling reflex testing, and work as an MDT to ensure biomarker testing is performed on all eligible patients with advanced NSCLC.17
[Abbreviations]
AA, African American; ALK, anaplastic lymphoma kinase; BRAF, proto-oncogene B-Raf; cfDNA, cell-free DNA; ctDNA, circulating tumor DNA; EGFR, epidermal growth factor receptor; EMR, electronic medical record; ERBB2, erb-b2 receptor tyrosine kinase 2; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; KRAS, Kirsten rat sarcoma viral oncogene homolog; MET, mesenchymal-to-epithelial transition; mNSCLC, metastatic non-small cell lung cancer; NSCLC, non-small cell lung cancer; NCCN, National Comprehensive Cancer Network; NGS, next-generation sequencing; NTRK, neurotrophic tyrosine receptor kinase; PD-L1, programmed cell death ligand 1; RET, rearranged during transfection; ROS1, c-ros oncogene 1; SOC, standard-of-care.
[References]
1. Majeed U, et al. J Hematol Oncol. 2021;14:108.
2. Sung H, et al. CA Cancer J Clin. 2021;71:209-249.
3. Siegel RL, et al. CA Cancer J Clin. 2021;71:7-33.
4. Food and Drug Administration. www.fda.gov. Accessed October 6, 2021.
5. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer. V.3.2022. ©National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed March 16, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
6. Pennell NA, et al. Am Soc Clin Oncol Educ Book. 2019;39:531-542.
7. Robert NJ, et al. Presented at: The American Society of Clinical Oncology Annual Meeting; June 4–8, 2021; Virtual Meeting. Abstract 102.
8. John A, et al. Adv Ther. 2021;38:1552-1566.
9. Hanna N, et al. J Clin Oncol. 2017;35:3484-3515.
10. Lindeman NI, et al. Arch Pathol Lab Med. 2018;142:321-346.
11. Nadler ES, et al. Presented at: The American Society of Clinical Oncology Annual Meeting; June 4–8, 2021; Virtual Meeting. Abstract 9079.
12. Bruno DS, et al. Presented at: The American Society of Clinical Oncology Annual Meeting; June 4–8, 2021; Virtual Meeting. Abstract 9005.
13. Hann KEJ, et al. BMC Public Health. 2017;17:503.
14. Pennell NA, et al. JCO Precis Oncol. 2019;3:1-9.
15. Rolfo C, et al. J Thorac Oncol. 2021;16:1647-1662.
16. Leighl NB, et al. Clin Cancer Res. 2019;25:4691-4700.
17. Gregg JP, et al. Transl Lung Cancer Res. 2019;8:286-301.
18. Smeltzer MP, et al. J Thorac Oncol. 2020;15:1434-1448.
USA-510-80864 02/22