Nutrition in Cancer Care (PDQ®)–Patient Version
Overview of Nutrition in Cancer Care
Key Points
- Good nutrition is important for people with cancer.
- Nutrition goals are set for each person with cancer.
- A registered dietitian is an important part of the healthcare team.
- Cancer and cancer treatments may cause side effects that affect nutrition.
- Cancer and cancer treatments may cause malnutrition.
- Anorexia and cachexia are common causes of malnutrition in people with cancer.
Good nutrition is important for people with cancer.
Nutrition is a process in which food is taken in and used by the body for growth, to keep the body healthy, and to replace tissue. Good nutrition is important for good health. A healthy diet includes foods and liquids that have important nutrients (vitamins, minerals, proteins, carbohydrates, fats, and water) the body needs.
Nutrition goals are set for each person with cancer.
Nutrition goals during cancer therapy are based on a person’s cancer type, cancer stage, and other medical conditions. Eating the right amount of protein and calories is important for healing, fighting infection, and having enough energy.
A registered dietitian is an important part of the healthcare team.
A registered dietitian (or nutritionist) is a part of the team of health professionals that help with cancer treatment and recovery. A dietitian will work with you, your family, and the rest of the medical team to manage your diet during and after cancer treatment.
Cancer and cancer treatments may cause side effects that affect nutrition.
Nutrition problems are likely when tumors involve the head, neck, esophagus, stomach, intestines, pancreas, or liver.
For many people, the effects of cancer treatments make it hard to eat well. Cancer treatments that affect nutrition include:
Cancer and cancer treatments may cause malnutrition.
Cancer and cancer treatments may affect taste, smell, appetite, and the ability to eat enough food or absorb the nutrients from food. This can cause malnutrition, which is a condition caused by a lack of key nutrients.
Malnutrition can cause a person to be weak, tired, and unable to fight infection or finish cancer treatment. As a result, malnutrition can decrease the person’s quality of life and become life-threatening. Malnutrition may get worse if the cancer grows or spreads.
Anorexia and cachexia are common causes of malnutrition in people with cancer.
Anorexia is the loss of appetite or desire to eat. It is a common symptom and the most common cause of malnutrition in people with cancer. Anorexia may occur early in the disease or later, if the cancer grows or spreads. Some people already have anorexia when they are diagnosed with cancer. Most people who have advanced cancer will have anorexia.
Cachexia is a condition marked by weakness, weight loss, and fat and muscle loss. It is common in people with tumors that affect eating and digestion. It can occur in people with cancer who are eating well, but are not storing fat and muscle because of tumor growth.
Some tumors change the way the body uses certain nutrients. The body’s use of protein, carbohydrates, and fat may change when tumors are in the stomach, intestines, or head and neck. A person may seem to be eating enough, but the body may not be able to absorb all the nutrients from the food.
People with cancer may have cachexia and anorexia at the same time (CAS), causing weight loss and decreased lean body mass. Treating high-risk patients to prevent this condition, rather than treating those already diagnosed with CAS, may lead to better outcomes. Olanzapine, a drug used to treat certain mental disorders, has side effects including increased appetite and weight gain. It is being studied in the treatment of CAS with mixed success. More clinical trials are needed to develop the best possible therapies for CAS.
Effects of Cancer Treatment on Nutrition
Key Points
- Chemotherapy and Hormone Therapy
- Chemotherapy and hormone therapy affect nutrition in different ways.
- Chemotherapy and hormone therapy cause different nutrition problems.
- Radiation Therapy
- Radiation therapy kills cells in the treatment area.
- Radiation therapy may affect nutrition.
- Surgery
- Surgery increases the body’s need for nutrients and energy.
- Surgery to the head, neck, esophagus, stomach, or intestines may affect nutrition.
- Immunotherapy
- Immunotherapy may affect nutrition.
- Stem Cell Transplant
- People who receive a stem cell transplant have special nutrition needs.
Chemotherapy and Hormone Therapy
Chemotherapy and hormone therapy affect nutrition in different ways.
Chemotherapy affects cells all through the body. Chemotherapy uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. Healthy cells that normally grow and divide quickly may also be killed. These include cells in the mouth and digestive tract.
Hormone therapy adds, blocks, or removes hormones. It may be used to slow or stop the growth of certain cancers. Some types of hormone therapy may cause weight gain.
Chemotherapy and hormone therapy cause different nutrition problems.
Side effects from chemotherapy may cause problems with eating and digestion. When more than one chemotherapy drug is given, each drug may cause different side effects, or when drugs cause the same side effect, the side effect may be more severe.
The following side effects are common:
People who receive hormone therapy may need changes in their diet to prevent weight gain.
Radiation Therapy
Radiation therapy kills cells in the treatment area.
Radiation therapy kills cancer cells and healthy cells in the treatment area. How severe the side effects are depends on the following:
Radiation therapy may affect nutrition.
Radiation therapy to any part of the digestive system has side effects that cause nutrition problems. Most of the side effects begin two to three weeks after radiation therapy begins and go away a few weeks after it is finished. Some side effects can continue for months or years after treatment ends.
The following are some of the more common side effects:
- For radiation therapy to the brain or head and neck
- Loss of appetite.
- Nausea.
- Vomiting.
- Dry mouth or thick saliva. Medication may be given to treat a dry mouth.
- Sore mouth and gums.
- Changes in the way food tastes.
- Trouble swallowing.
- Pain when swallowing.
- Being unable to fully open the mouth.
- For radiation therapy to the chest
- Loss of appetite.
- Nausea.
- Vomiting.
- Trouble swallowing.
- Pain when swallowing.
- Choking or breathing problems caused by changes in the upper esophagus.
- For radiation therapy to the abdomen, pelvis, or rectum
- Nausea.
- Vomiting.
- Bowel obstruction.
- Colitis.
- Diarrhea.
Radiation therapy may also cause tiredness, which can lead to a decrease in appetite.
Surgery
Surgery increases the body’s need for nutrients and energy.
The body needs extra energy and nutrients to heal wounds, fight infection, and recover from surgery. If someone is malnourished before surgery, they may have trouble healing. For these people, nutrition care may begin before surgery.
Surgery to the head, neck, esophagus, stomach, or intestines may affect nutrition.
Most people with cancer are treated with surgery. Surgery that removes all or part of certain organs can affect a person’s ability to eat and digest food.
The following are nutrition problems caused by surgery:
- Loss of appetite.
- Trouble chewing.
- Trouble swallowing.
- Feeling full after eating a small amount of food.
Immunotherapy
Immunotherapy may affect nutrition.
The side effects of immunotherapy are different for each person and the type of immunotherapy drug given.
The following nutrition problems are common:
- Fatigue.
- Fever.
- Nausea.
- Vomiting.
- Diarrhea.
Stem Cell Transplant
People who receive a stem cell transplant have special nutrition needs.
Chemotherapy, radiation therapy, and other medicines used before or during a stem cell transplant may cause side effects that keep a person from eating and digesting food as usual.
Common side effects include the following:
- Mouth and throat sores.
- Diarrhea.
People who receive a stem cell transplant have a high risk of infection. Chemotherapy or radiation therapy given before the transplant decrease the number of white blood cells, which fight infection. It is important that these people learn about safe food handling and avoid foods that may cause infection.
After a stem cell transplant, people are at risk for acute or chronic graft-versus-host disease (GVHD). GVHD may affect the gastrointestinal tract or liver and change the person’s ability to eat or absorb nutrients from food.
Nutrition Assessment in Cancer Care
Key Points
- The healthcare team may ask questions about diet and weight history.
- Counseling and diet changes are made to improve the person’s nutrition.
- The goal of nutrition therapy for people who have advanced cancer depends on the overall plan of care.
The healthcare team may ask questions about diet and weight history.
Screening is used to look for health problems that affect the risk of poor nutrition. This can help find out if you are likely to become malnourished, and if nutrition therapy is needed.
The healthcare team may ask questions about the following:
- Weight changes over the past year.
- Changes in the amount and type of food you’ve eaten.
- Problems with eating, such as loss of appetite, nausea, vomiting, diarrhea, constipation, mouth sores, dry mouth, changes in taste and smell, or pain.
- Ability to walk and do other activities of daily living (dressing, getting into or out of a bed or chair, taking a bath or shower, and using the toilet).
A physical exam is done. Your doctor will check for signs of weight, fat, and muscle loss, and for fluid buildup in your body.
Counseling and diet changes are made to improve the person’s nutrition.
A registered dietitian can counsel you and your family on ways to improve your nutrition. The registered dietitian gives care based on your nutritional and dietary needs during cancer treatment and recovery. Changes to the diet are made to help decrease symptoms from cancer or cancer treatment. These changes may be in the types and amount of food, how often you eat, and how food is eaten (for example, at a certain temperature or taken with a straw).
In addition to the dietitian, the healthcare team may include the following:
The goal of nutrition therapy for people who have advanced cancer depends on the overall plan of care.
The goal of nutrition therapy in people with advanced cancer is to provide the best possible quality of life and control symptoms that cause distress.
People with advanced cancer may be treated with anticancer therapy and palliative care, palliative care alone, or may be in hospice care. Nutrition goals will be different for each person. Some types of treatment may be stopped.
As the focus of care goes from cancer treatment to hospice or end-of-life care, nutrition therapy may become less aggressive to keep the person as comfortable as possible. For more information, see the Nutrition Needs at End of Life section.
Treatment of Symptoms
Key Points
- Anorexia
- Nausea
- Vomiting
- Dry Mouth
- Mouth Sores
- Taste Changes
- Sore Throat and Trouble Swallowing
- Lactose Intolerance
- Weight Gain
When side effects of cancer or cancer treatment affect normal eating, changes can be made to help you get the nutrients you need. Eating foods that are high in calories, protein, vitamins, and minerals is important. Meals should be planned to meet your nutritional needs and tastes in food.
The following are common symptoms caused by cancer and cancer treatment and ways to treat or control them.
Anorexia
The following may help people with cancer who have anorexia (loss of appetite or desire to eat):
- Eat foods that are high in protein and calories. The following are high-protein food choices:
- Beans.
- Chicken.
- Fish.
- Meat.
- Yogurt.
- Eggs.
- Add extra protein and calories to food, such as using protein-fortified milk.
- Eat high-protein foods first in your meal when your appetite is strongest.
- Sip only small amounts of liquids during meals.
- Drink milkshakes, smoothies, juices, or soups if you do not feel like eating solid foods.
- Eat foods that smell good.
- Try new foods and new recipes.
- Try blenderized drinks that are high in nutrients (check with your doctor or registered dietitian first).
- Eat small meals and healthy snacks often throughout the day.
- Eat larger meals when you feel well and are rested.
- Eat your largest meal when you feel hungriest, whether at breakfast, lunch, or dinner.
- Make and store small amounts of favorite foods so they are ready to eat when you are hungry.
- Be as active as possible so that you will have a good appetite.
- Brush your teeth and rinse your mouth to relieve symptoms and aftertastes.
- Talk to your doctor or registered dietitian if you have eating problems such as nausea, vomiting, or changes in how foods taste and smell.
If these diet changes do not help with the anorexia, tube feedings may be needed.
Medicines may be given to increase appetite. For more information, see the Medicines to Treat Loss of Appetite and Weight Loss section.
Nausea
The following may help people with cancer control nausea:
- Choose foods that appeal to you. Do not force yourself to eat food that makes you feel sick. Do not eat your favorite foods, to avoid linking them to being sick.
- Eat foods that are bland, soft, and easy-to-digest, rather than heavy meals.
- Eat dry foods such as crackers, bread sticks, or toast throughout the day.
- Eat foods that are easy on your stomach, such as white toast, plain yogurt, and clear broth.
- Eat dry toast or crackers before getting out of bed if you have nausea in the morning.
- Eat foods and drink liquids at room temperature (not too hot or too cold).
- Slowly sip liquids throughout the day.
- Suck on hard candies such as peppermints or lemon drops if your mouth has a bad taste.
- Stay away from foods and drinks with strong smells.
- Eat 5 or 6 small meals every day instead of 3 large meals.
- Sip on only small amounts of liquid during meals to avoid feeling full or bloated.
- Do not skip meals and snacks. An empty stomach may make your nausea worse.
- Rinse your mouth before and after eating.
- Don’t eat in a room that has cooking odors or that is very warm. Keep the living space at a comfortable temperature and well-ventilated.
- Sit up or lie with your head raised for one hour after eating.
- Plan the best times for you to eat and drink.
- Relax before each cancer treatment.
- Wear clothes that are loose and comfortable.
- Keep a record of when you feel nausea and why.
- Talk with your doctor about using antinausea medicine.
Vomiting
The following may help people with cancer control vomiting:
- Do not eat or drink anything until the vomiting stops.
- Drink small amounts of clear liquids after vomiting stops.
- After you are able to drink clear liquids without vomiting, drink liquids such as strained soups, or milkshakes, that are easy on your stomach.
- Eat 5 or 6 small meals every day instead of 3 large meals.
- Sit upright and bend forward after vomiting.
- Ask your doctor to order medicine to prevent or control vomiting.
Dry Mouth
The following may help people with cancer who have dry mouth:
- Eat foods that are easy to swallow.
- Moisten food with sauce, gravy, or salad dressing.
- Eat foods and drinks that are very sweet or tart, such as lemonade, to help make more saliva.
- Chew gum or suck on hard candy, ice pops, or ice chips.
- Sip water throughout the day.
- Do not drink any type of alcohol, beer, or wine.
- Do not eat foods that can hurt your mouth (such as spicy, sour, salty, hard, or crunchy foods).
- Keep your lips moist with lip balm.
- Rinse your mouth every 1 to 2 hours. Do not use mouthwash that contains alcohol.
- Do not use tobacco products and avoid second hand smoke.
- Ask your doctor or dentist about using artificial saliva or similar products to coat, protect, and moisten your mouth and throat.
Mouth Sores
The following can help people with cancer who have mouth sores:
- Eat soft foods that are easy to chew, such as milkshakes, scrambled eggs, and custards.
- Cook foods until soft and tender.
- Cut food into small pieces. Use a blender or food processor to make food smooth.
- Suck on ice chips to numb and soothe your mouth.
- Eat foods cold or at room temperature. Hot foods can hurt your mouth.
- Drink with a straw to move liquid past the painful parts of your mouth.
- Use a small spoon to help you take smaller bites, which are easier to chew.
- Stay away from the following:
- Citrus foods, such as oranges, lemons, and limes.
- Spicy foods.
- Tomatoes and ketchup.
- Salty foods.
- Raw vegetables.
- Sharp and crunchy foods.
- Drinks with alcohol.
- Do not use tobacco products.
- Visit a dentist at least 2 weeks before starting immunotherapy, chemotherapy, or radiation therapy to the head and neck.
- Check your mouth each day for sores, white patches, or puffy and red areas.
- Rinse your mouth 3 to 4 times a day. Mix ¼ teaspoon baking soda, ⅛ teaspoon salt, and 1 cup warm water for a mouth rinse. Do not use mouthwash that contains alcohol.
- Do not use toothpicks or other sharp objects.
Taste Changes
The following may help people with cancer who have taste changes:
- Eat poultry, fish, eggs, and cheese instead of red meat.
- Add spices and sauces to foods (marinate foods).
- Eat meat with something sweet, such as cranberry sauce, jelly, or applesauce.
- Try tart foods and drinks.
- Use sugar-free lemon drops, gum, or mints if there is a metallic or bitter taste in your mouth.
- Use plastic utensils and do not drink directly from metal containers if foods have a metal taste.
- Try to eat your favorite foods, if you are not nauseated. Try new foods when feeling your best.
- Find nonmeat, high-protein recipes in a vegetarian or Chinese cookbook.
- Chew food longer to allow more contact with taste buds, if food tastes dull but not unpleasant.
- Keep foods and drinks covered, drink through a straw, turn a kitchen fan on when cooking, or cook outdoors if smells bother you.
- Brush your teeth and take care of your mouth. Visit your dentist for checkups.
Sore Throat and Trouble Swallowing
The following may help people with cancer who have a sore throat or trouble swallowing:
- Eat soft foods that are easy to chew and swallow, such as milkshakes, scrambled eggs, oatmeal, or other cooked cereals.
- Eat foods and drinks that are high in protein and calories.
- Moisten food with gravy, sauces, broth, or yogurt.
- Stay away from the following foods and drinks that can burn or scratch your throat:
- Hot foods and drinks.
- Spicy foods.
- Foods and juices that are high in acid.
- Sharp or crunchy foods.
- Drinks with alcohol.
- Cook foods until soft and tender.
- Cut food into small pieces. Use a blender or food processor to make food smooth.
- Drink with a straw.
- Eat 5 or 6 small meals every day instead of 3 large meals.
- Sit upright and bend your head slightly forward when you eat or drink, and stay upright for at least 30 minutes after eating.
- Do not use tobacco.
- Talk to your doctor about tube feedings if you cannot eat enough to stay strong.
Lactose Intolerance
The following may help people with cancer who have symptoms of lactose intolerance:
- Use lactose-free or low-lactose milk products. Most grocery stores carry food (such as milk and ice cream) labeled “lactose free” or “low lactose.”
- Choose milk products that are low in lactose, like hard cheeses (such as cheddar) and yogurt.
- Try products made with soy or rice (such as soy and rice milk and frozen desserts). These products do not contain lactose.
- Avoid only the dairy products that give you problems. Eat small portions of dairy products, such as milk, yogurt, or cheese, if you can.
- Try nondairy drinks and foods with calcium added.
- Eat calcium-rich vegetables, such as broccoli and greens.
- Take lactase tablets when eating or drinking dairy products. Lactase breaks down lactose, so it is easier to digest.
- Prepare your own low-lactose or lactose-free foods.
Weight Gain
The following may help people with cancer prevent weight gain:
- Eat a lot of fruits and vegetables.
- Eat foods that are high in fiber, such as whole-grain breads, cereals, and pasta.
- Choose lean meats, such as lean beef, pork trimmed of fat, and poultry (such as chicken or turkey) without skin.
- Choose low-fat milk products.
- Eat less fat (eat only small amounts of butter, mayonnaise, desserts, and fried foods).
- Cook with low-fat methods, such as broiling, steaming, grilling, or roasting.
- Eat less salt.
- Eat foods that you enjoy so you feel satisfied.
- Eat only when hungry. Consider counseling or medicine if you eat because of stress, fear, or depression. If you eat because you are bored, find activities you enjoy.
- Eat smaller amounts of food at meals.
- Exercise daily.
- Talk with your doctor before going on a diet to lose weight.
Types of Nutrition Support
Key Points
- Nutrition support helps people who cannot eat or digest food normally.
- Nutrition support can be given in different ways.
- Enteral Nutrition
- Enteral nutrition is also called tube feeding.
- Parenteral Nutrition
- Parenteral nutrition carries nutrients directly into the blood stream.
- The catheter may be placed into a vein in the chest or in the arm.
Nutrition support helps people who cannot eat or digest food normally.
It is best to take in food by mouth whenever possible. Some people may not be able to take in enough food by mouth because of problems from cancer or cancer treatment.
Nutrition support can be given in different ways.
In addition to counseling by a dietitian and changes to the diet, nutrition therapy includes nutritional supplement drinks and enteral and parenteral nutrition support. Nutritional supplement drinks help people with cancer get the nutrients they need. They provide energy, protein, fat, carbohydrates, fiber, vitamins, and minerals. They are not meant to be the person’s only source of nutrition.
A person who is not able to take in the right amount of calories and nutrients by mouth may be fed using the following:
- Enteral nutrition: Nutrients are given through a tube inserted into the stomach or intestines.
- Parenteral nutrition: Nutrients are infused into the bloodstream.
Nutrition support can improve a person’s quality of life during cancer treatment, but may cause problems that should be considered before making the decision to use it. The patient, family, and healthcare team should discuss the harms and benefits of each type of nutrition support. For more information on the use of nutrition support at the end of life, see the Nutrition Needs at End of Life section.
Enteral Nutrition
Enteral nutrition is also called tube feeding.
Enteral nutrition gives the patient nutrients in liquid form (formula) through a tube that is placed into the stomach or small intestine. The following types of feeding tubes may be used:
- A nasogastric tube is inserted through the nose and down the throat into the stomach or small intestine. This is used when enteral nutrition is only needed for a few weeks.
- A gastrostomy tube is inserted into the stomach, or a jejunostomy tube is inserted into the small intestine through an opening made on the outside of the abdomen. This is usually used for long-term enteral feeding or for people who cannot use a tube in the nose and throat.
The type of formula used is based on the person’s specific needs. There are formulas for people who have special health conditions, such as diabetes, or other needs, such as religious or cultural diets.
Parenteral Nutrition
Parenteral nutrition carries nutrients directly into the blood stream.
Parenteral nutrition is used when a person cannot take food by mouth or by enteral feeding. Parenteral feeding does not use the stomach or intestines to digest food. Nutrients are given to the patient directly into the blood, through a catheter inserted into a vein. These nutrients include proteins, fats, vitamins, and minerals.
The catheter may be placed into a vein in the chest or in the arm.
A central venous access catheter is placed beneath the skin and into a large vein in the upper chest. The catheter is put in place by a surgeon. This type of catheter is used for long-term parenteral feeding.
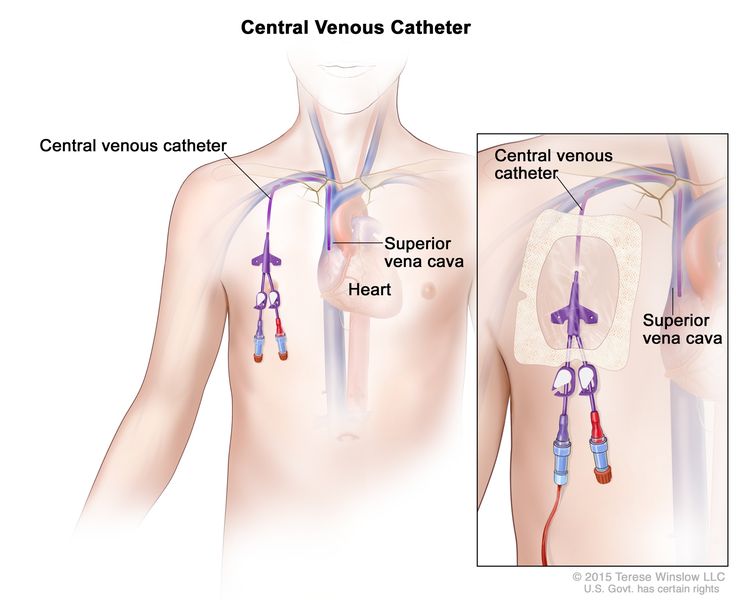
A peripheral venous catheter is placed into a vein in the arm. A peripheral venous catheter is put in place by trained medical staff. This type of catheter is usually used for short-term parenteral feeding for patients who do not have a central venous access catheter.
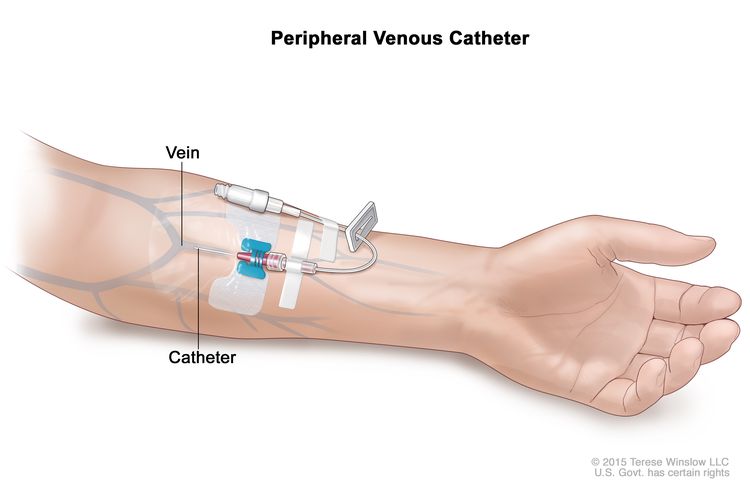
The patient is checked often for infection or bleeding at the place where the catheter enters the body.
Medicines to Treat Loss of Appetite and Weight Loss
Key Points
- Medicine may be given with nutrition therapy to treat loss of appetite and weight loss.
- Different types of medicine may be used to treat loss of appetite and weight loss.
Medicine may be given with nutrition therapy to treat loss of appetite and weight loss.
It is important that cancer symptoms and side effects that affect eating and cause weight loss are treated early. Both nutrition therapy and medicine can help lessen the effects that cancer and its treatment have on weight loss.
Different types of medicine may be used to treat loss of appetite and weight loss.
Medicines that improve appetite and cause weight gain, such as prednisone and megestrol, may be used to treat loss of appetite and weight loss. Studies have shown that the effects of these medicines may not last long, or there may be no effects. Treatment with a combination of medicines may work better than treatment with one medicine but may have more side effects.
Nutrition Needs at End of Life
Key Points
- Nutrition needs change at end of life.
- People with cancer and their families decide how much nutrition and fluids will be given at the end of life.
Nutrition needs change at end of life.
For people at the end of life, the goals of nutrition therapy are focused on relieving symptoms rather than getting enough nutrients.
Common symptoms that can occur at the end of life include the following:
People who have problems swallowing may find it easier to swallow thick liquids than thin liquids.
People with cancer often do not feel much hunger at all and may want very little food. Sips of water, ice chips, and mouth care can decrease thirst in the last few days of life. Good communication with the healthcare team is important to understand the patient’s changes in nutrition needs.
People with cancer and their families decide how much nutrition and fluids will be given at the end of life.
People with cancer and their caregivers have the right to make informed decisions. The person’s religious and cultural preferences may affect their decisions. The healthcare team may work with the person’s religious and cultural leaders when making decisions. The healthcare team and a registered dietitian can explain the benefits and risks of using nutrition support for people at the end of life. In most cases, there are more harms than benefits if the person is not expected to live longer than a month.
Possible benefits of nutrition support for people expected to live longer than a month include the following:
- Improved quality of life.
- Less risk of death due to malnutrition.
- Fewer physical, mental, and psychological problems.
The risks of nutrition support at the end of life include the following:
- Sepsis (bacteria or their toxins in the blood or tissues) with the use of parenteral nutrition.
- Aspiration (the accidental breathing in of food or fluid into the lungs) with the use of enteral nutrition.
- Sores and breakdown of the skin where the enteral feeding tube is inserted.
- Diarrhea with the use of enteral and parenteral nutrition.
- Complications caused by fluid overload (a condition where there is too much fluid in the blood) with the use of enteral and parenteral nutrition.
Nutrition Trends in Cancer
Key Points
- Some people with cancer try special diets to improve their prognosis.
- Some people with cancer may take dietary supplements.
Some people with cancer try special diets to improve their prognosis.
People with cancer may try special diets to make their treatment work better, prevent side effects from treatment, or to treat the cancer itself. However, for most of these special diets, there is no evidence that shows they work.
Vegetarian or vegan diet
It is not known if following a vegetarian or vegan diet can help side effects from cancer treatment or the person’s prognosis. If the person already follows a vegetarian or vegan diet, there is no evidence that shows they should switch to a different diet.
A study in patients with non-muscle-invasive bladder cancer showed some benefits from eating a diet rich in ITC, a phytochemical found in raw cruciferous vegetables. Patients who ate large amounts of cruciferous vegetables were less likely to have two or more recurrences of their disease and a lower risk of their disease becoming muscle-invasive cancer. More research on the benefits of phytochemicals is needed.
Macrobiotic diet
A macrobiotic diet is a high-carbohydrate, low-fat, plant-based diet. No studies have shown that this diet will help people with cancer.
Ketogenic diet
A ketogenic diet limits carbohydrates and increases fat intake. The purpose of the diet is to decrease the amount of glucose (sugar) the tumor cells can use to grow and reproduce. It is a hard diet to follow because exact amounts of fats, carbohydrates, and proteins are needed.
Several clinical trials are recruiting people with glioblastoma to study whether a ketogenic diet affects glioblastoma tumor activity. People with glioblastoma who want to start a ketogenic diet should talk to their doctor and work with a registered dietitian. However, it is not yet known how the diet will affect the tumor or its symptoms.
Similarly, a study comparing the ketogenic diet to a high-fiber, low fat diet in women with ovarian cancer or endometrial cancer found that the ketogenic diet was safe and acceptable. There is not enough evidence to know how the ketogenic diet will affect ovarian or endometrial tumors or their symptoms.
Some people with cancer may take dietary supplements.
A dietary supplement is a product that is added to the diet. It is usually taken by mouth, and usually has one or more dietary ingredients. People with cancer may take dietary supplements to improve their symptoms or treat their cancer.
Vitamin C
Vitamin C is a nutrient that the body needs in small amounts to function and stay healthy. It helps fight infection, heal wounds, and keep tissues healthy. Vitamin C is found in fruits and vegetables. It can also be taken as a dietary supplement. For information about the use of intravenous vitamin C as treatment for people with cancer, see Intravenous Vitamin C.
Probiotics
Probiotics are live microorganisms used as dietary supplements to help with digestion and normal bowel function. They may also help keep the gastrointestinal tract healthy.
Studies have shown that taking probiotics during radiation therapy and chemotherapy can help prevent diarrhea caused by those treatments. People with cancer who are receiving radiation therapy to the abdomen or chemotherapy that is known to cause diarrhea may be helped by probiotics. Similarly, studies are looking at potential benefits of taking probiotics for people with cancer who are receiving immunotherapy.
Melatonin
Melatonin is a hormone made by the pineal gland (tiny organ near the center of the brain). Melatonin helps control the body’s sleep cycle. It can also be made in a laboratory and taken as a dietary supplement.
Several small studies have shown that taking a melatonin supplement with chemotherapy and/or radiation therapy for treatment of solid tumors may be helpful. It may help reduce side effects of treatment. Melatonin does not appear to have side effects.
Oral glutamine
Oral glutamine is an amino acid that is being studied for the treatment of diarrhea and mucositis (inflammation of the lining of the digestive system, often seen as mouth sores) caused by chemotherapy or radiation therapy. Oral glutamine may help prevent mucositis or make it less severe.
People with cancer who are receiving radiation therapy to the abdomen may benefit from oral glutamine. Oral glutamine may reduce the severity of diarrhea. This can help people continue with their treatment plan.
To Learn More About Nutrition and Cancer Care
National Cancer Institute
For information from the National Cancer Institute (NCI) about nutrition and cancer treatment, see Side Effects.
Organizations
For general nutrition information and other resources, see the following:
- United States Department of Agriculture
- Academy of Nutrition and Dietetics
- 800-877-1600
- www.eatright.org
- American Botanical Council
- 800-373-7105
- abc.herbalgram.org
- American Cancer Society
- 800-227-2345
- www.cancer.org/healthy/eat-healthy-get-active
- American Institute for Cancer Research
- 800-843-8114
- www.aicr.org/cancer-prevention/healthy-eating
- American Society for Parenteral and Enteral Nutrition
- 301-587-6315
- www.nutritioncare.org
- National Center for Complementary and Integrative Health (NCCIH)
- 888-644-6226 (NCCIH Clearinghouse)
- 866-464-3615 (toll free TTY)
- nccih.nih.gov
- Office of Dietary Supplements
- 301-435-2920
- ods.od.nih.gov
Books
- American Cancer Society’s Healthy Eating Cookbook: A Celebration of Food, Friends, and Healthy Living. 3rd ed. Atlanta, GA: The American Cancer Society, 2005.
- Bloch A, Cassileth BR, Holmes MD, Thomson CA, eds.: Eating Well, Staying Well During and After Cancer. Atlanta, GA: American Cancer Society, 2004.
- Ghosh K, Carson L, and Cohen E: Betty Crocker’s Living with Cancer Cookbook: Easy Recipes and Tips Through Treatment and Beyond. New York, NY: Hungry Minds, 2002.
- Weihofen DL, Robbins J, Sullivan PA: Easy-to-Swallow, Easy-to-Chew Cookbook: Over 150 Tasty and Nutritious Recipes for People Who Have Difficulty Swallowing. New York, NY: John Wiley & Sons, Inc., 2002.
- Wilson JR: I-Can’t-Chew Cookbook: Delicious Soft Diet Recipes for People with Chewing, Swallowing, or Dry Mouth Disorders. Alameda, Calif: Hunter House Inc., 2003.
Current Clinical Trials
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
About This PDQ Summary
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute’s (NCI’s) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about nutrition before, during, and after cancer treatment. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary (“Updated”) is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Supportive and Palliative Care Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become “standard.” Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI’s website. For more information, call the Cancer Information Service (CIS), NCI’s contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Supportive and Palliative Care Editorial Board. PDQ Nutrition in Cancer Care. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: /side-effects/appetite-loss/nutrition-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389440]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us.

